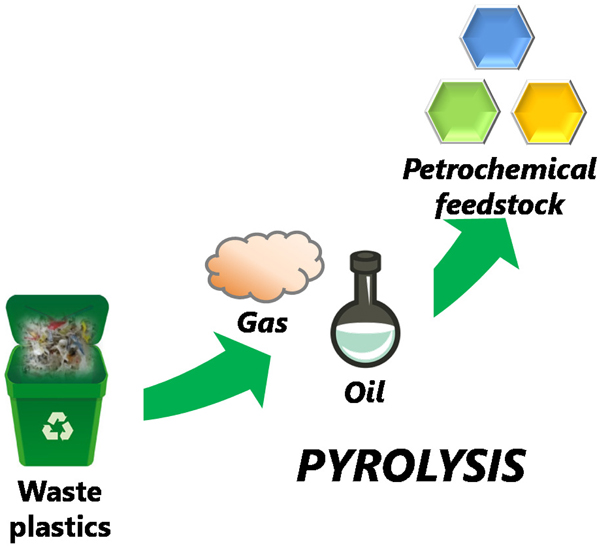
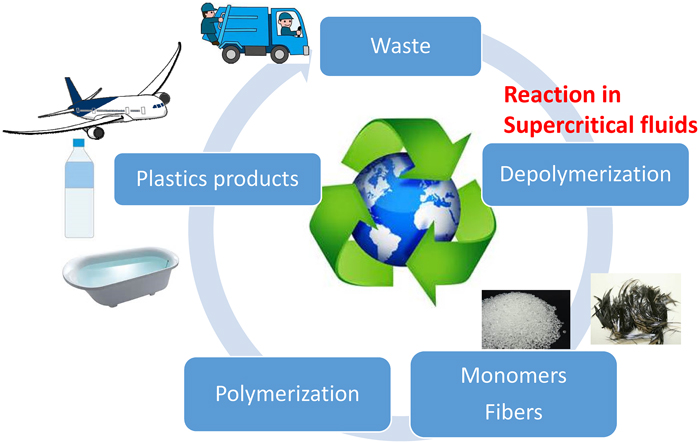
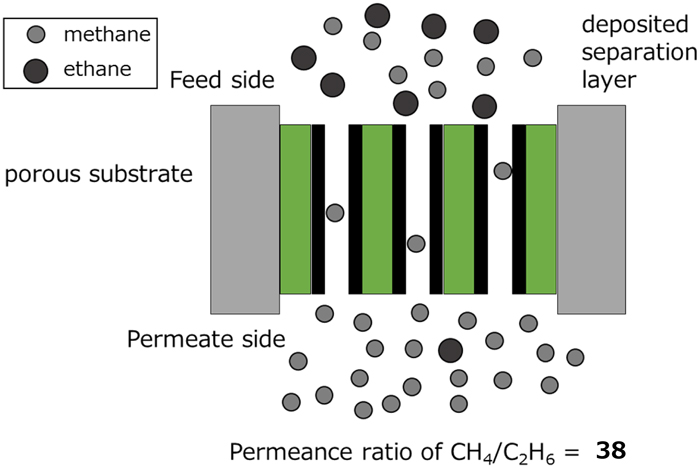
Volume 59, Issue 6
Displaying 1-11 of 11 articles from this issue
- |<
- <
- 1
- >
- >|
Review Paper
-
Article type: Review Paper
2016 Volume 59 Issue 6 Pages 243-253
Published: November 01, 2016
Released on J-STAGE: January 01, 2017
Download PDF (4385K) -
Article type: Review Paper
2016 Volume 59 Issue 6 Pages 254-258
Published: November 01, 2016
Released on J-STAGE: January 01, 2017
Download PDF (1432K)
Review Paper — Feature Articles: Preparation of Porous Membrane and Application to Separation —
-
Article type: Review Paper
2016 Volume 59 Issue 6 Pages 259-265
Published: November 01, 2016
Released on J-STAGE: January 01, 2017
Download PDF (971K) -
Article type: Review Paper
2016 Volume 59 Issue 6 Pages 266-275
Published: November 01, 2016
Released on J-STAGE: January 01, 2017
Download PDF (5053K) -
Article type: Review Paper
2016 Volume 59 Issue 6 Pages 276-282
Published: November 01, 2016
Released on J-STAGE: January 01, 2017
Download PDF (3640K)
Regular Paper
-
Article type: Regular Paper
2016 Volume 59 Issue 6 Pages 283-292
Published: November 01, 2016
Released on J-STAGE: January 01, 2017
Download PDF (2133K) -
Article type: Regular Paper
2016 Volume 59 Issue 6 Pages 293-298
Published: November 01, 2016
Released on J-STAGE: January 01, 2017
Download PDF (1022K) -
Article type: Regular Paper
2016 Volume 59 Issue 6 Pages 299-306
Published: November 01, 2016
Released on J-STAGE: January 01, 2017
Download PDF (835K)
Technical Report
-
Article type: Technical Report
2016 Volume 59 Issue 6 Pages 307-310
Published: November 01, 2016
Released on J-STAGE: January 01, 2017
Download PDF (529K) -
Article type: Technical Report
2016 Volume 59 Issue 6 Pages 311-316
Published: November 01, 2016
Released on J-STAGE: January 01, 2017
Download PDF (582K) -
Article type: Technical Report
2016 Volume 59 Issue 6 Pages 317-321
Published: November 01, 2016
Released on J-STAGE: January 01, 2017
Download PDF (349K)
- |<
- <
- 1
- >
- >|