
- Issue 6 Pages 263-
- Issue 5 Pages 203-
- Issue 4 Pages 159-
- Issue 3 Pages 113-
- Issue 2 Pages 63-
- Issue 1 Pages 1-
- |<
- <
- 1
- >
- >|
-
Masaaki Kitano, Hisayoshi Kobayashi, Shigenobu Hayashi, Michikazu HaraArticle type: Review Paper
2017 Volume 60 Issue 3 Pages 113-120
Published: May 01, 2017
Released on J-STAGE: May 01, 2017
JOURNAL FREE ACCESSAcid properties of protonated titanate nanotubes were investigated by comparing the catalytic properties of various titanate materials with different morphology. Titanate nanotubes with a scroll-like layered structure exhibited excellent catalytic performance for Friedel-Crafts alkylation and Beckmann rearrangement of cyclohexanone, exceeding the activities of titanate nanorods and nanosheets with similar crystal structures to that of the titanate nanotubes. The ion-exchange capacity of titanate nanorods is much larger than that of titanate nanotubes, but the ion-exchange sites (i.e. Brønsted acid sites) of titanate nanorods are located at the interspace of layered structures and strongly interact with the anionic titanate layers, i.e., the Brønsted acid sites are not exposed on the surface. FT-IR analyses and ion-exchange experiments revealed that titanate nanotubes possess both Brønsted and Lewis acid sites on the catalyst surface. In addition, the Brønsted acidity of titanate nanotubes is much higher than that of titanate nanosheets due to lattice distortion caused by scrolling of the lamellar titanate nanosheets. The synergic effect between Brønsted and Lewis acid sites is important in the high catalytic performance of titanate nanotubes for various acid-catalyzed reactions.
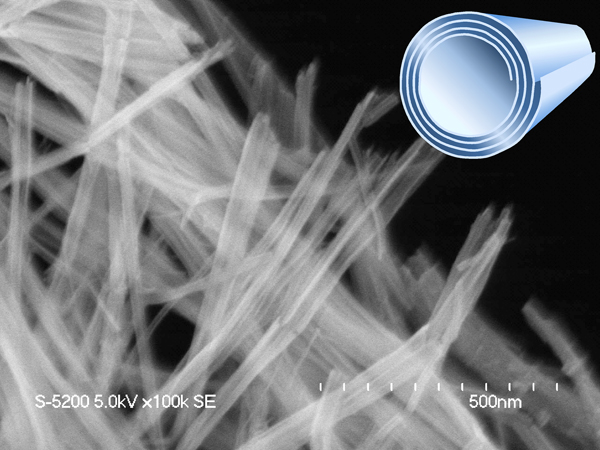 View full abstractDownload PDF (1710K)
View full abstractDownload PDF (1710K) -
Takeo Ebina, Ryo Ishii, Takafumi Aizawa, Hajime YoshidaArticle type: Review Paper
2017 Volume 60 Issue 3 Pages 121-126
Published: May 01, 2017
Released on J-STAGE: May 01, 2017
JOURNAL FREE ACCESSWe have recently developed a new composite material, a clay-based film (CBF), for hydrogen tanks, with excellent hydrogen gas barrier properties and high durability. Preliminary test results showed that the hydrogen gas barrier properties of this material were approximately two to three orders of magnitude better than those of other polymer materials. We further investigated the relationship between the elongation and gas barrier properties of nanocomposite films composed of nylon and Li-exchanged smectite. As a result, we proposed films with a 70 % clay content, excellent in terms of both their elongation at break and gas barrier properties. Finally, multi-layered materials composed of carbon fiber reinforced plastic (CFRP) and CBF, and CFRP-based tanks were prepared using CBF. The developed material was shown to be highly applicable to lightweight hydrogen tanks for use in aircrafts, spacecrafts, and vehicles.
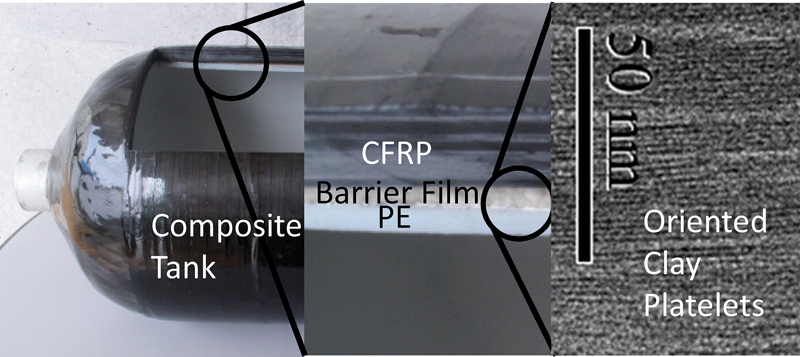 View full abstractDownload PDF (1909K)
View full abstractDownload PDF (1909K)
-
Masaru Saito, Hiroshi Nagasaki, Shigeyuki Watanabe, Takanori FujimotoArticle type: Regular Paper
2017 Volume 60 Issue 3 Pages 127-136
Published: May 01, 2017
Released on J-STAGE: May 01, 2017
JOURNAL FREE ACCESSFermentation of cellulosic and hemicellulosic sugars from biomass resources has potential to resolve food-versus-fuel conflicts. Inability to consume xylose and glucose simultaneously is one of the problems related to the economical use of lignocellulose as a feedstock. In this study, Candida intermedia strain NBRC 10601 was cultured with acetic acid as inhibitor at pH 5. C. intermedia 4-6-4T2 was isolated as an adapted mutant strain. C. intermedia 4-6-4T2 could efficiently convert both xylose and glucose to ethanol. The fermentation activity of C. intermedia 4-6-4T2 was compared with four other xylose-fermenting yeasts (C. intermedia 10601 (parental strain), Pichia stipitis, Candida shehatae and Pachysolen tannophilus) precultured in a rich medium with various concentrations of glucose and/or xylose as the carbon source. Except for P. tannophilus, the fermentation activity of these four yeast strains precultured with glucose plus xylose as the carbon source increased in an evaluative sugar solution adjusted to pH 5.5 containing 44 g/L xylose plus 88 g/L glucose with 3 g/L of acetic acid as the inhibitor. C. intermedia 4-6-4T2 produced the highest concentration of ethanol (54 g/L) among the tested strains and produced about 50 g/L of ethanol with ethanol productivity of 1.0 g/L/h from sugars using hydrolysate of sugarcane bagasse or corn stover.
 View full abstractDownload PDF (2363K)
View full abstractDownload PDF (2363K) -
Fumihiro Watanabe, Ikuko Kaburaki, Naohiro Shimoda, Akira Igarashi, Sh ...Article type: Regular Paper
2017 Volume 60 Issue 3 Pages 137-145
Published: May 01, 2017
Released on J-STAGE: May 01, 2017
JOURNAL FREE ACCESSSulfur tolerances of α-Al2O3 supported noble metal (Rh, Pt, Ir, and Ru) catalysts for steam methane reforming (SMR) at 700 °C were examined. Methane conversions over all catalysts decreased in the presence of dimethyl sulfide (DMS) as a sulfur contaminant in the SMR feed. Higher DMS concentration in the SMR feed accelerated the degradation rate of all catalysts. In general, sulfur poisoning is one of major reasons for catalyst degradation. The catalysts showed different degradation behaviors depending on the DMS concentration and the active metal species. Rh and Ru catalysts were deactivated completely within the first few hours of SMR with DMS. Methane conversions over Pt and Ir catalysts also decreased to some extent within hours, but subsequently remained unchanged. Pt and Ir catalysts poisoned by sulfur were fully regenerated after stopping the DMS feed, and Rh catalyst poisoned by sulfur recovered to some extent, whereas Ru catalyst poisoned by sulfur was little regenerated.
 View full abstractDownload PDF (1352K)
View full abstractDownload PDF (1352K) -
Gaku Watanabe, Yuta Nakasaka, Takao MasudaArticle type: Regular Paper
2017 Volume 60 Issue 3 Pages 146-153
Published: May 01, 2017
Released on J-STAGE: May 01, 2017
JOURNAL FREE ACCESSAluminosilicate and ferrisilicate with the MTW-type zeolite structure were hydrothermally prepared and used to catalyze the methylation of 2-methylnaphthalene (2-MN) under supercritical conditions in a batch-type reactor. Dimethylnaphthalene (DMN) and 1-methylnaphthalene (1-MN) yields increased with decreasing Si/Al ratio of the zeolite. In contrast, ferrisilicate with the MTW-type zeolite structure (Si/Fe=100) exhibited higher DMN selectivity and the β,β-DMN fraction in total DMNs was higher than that over aluminosilicate with MTW structure (Si/Al=100). Kinetic analysis of 2-MN methylation over MTW zeolites was conducted, to obtain the reaction rate constants and apparent activation energies. The activation energy for the methylation of 2-MN over aluminosilicate with MTW structure was 176 kJ mol−1, which was almost the same as that for the ferrisilicate with MTW structure. This indicates that the mechanism for methylation is independent of hetero-atoms species in the MTW zeolite framework. The introduction of Fe atoms into the framework significantly decreased the rate constant for the isomerization of 2-MN compared with that for the methylation of 2-MN. Therefore, ferrisilicate with MTW structure is effective for 2-MN methylation due to the decrease of the isomerization rate.
 View full abstractDownload PDF (782K)
View full abstractDownload PDF (782K)
-
Toru Matsui, Tomohiko NishinoArticle type: Research Note
2017 Volume 60 Issue 3 Pages 154-157
Published: May 01, 2017
Released on J-STAGE: May 01, 2017
JOURNAL FREE ACCESSImmobilization of alkane-degrading bacteria by physical adsorption on polyurethane foam support was examined for application to the repeated degradation of alkane. Presence of hydrophobic substrate during cultivation promoted the attachment of alkane-degrading bacteria, such as Brevibacterium, Rhodococcus, and Pseudomonas sp., possibly due to the absorption of hydrophobic substrate on the polyurethane foam, followed by bacterial attachment to the substrate. Efficiency of immobilization depended on the hydrophobicity index, log P of the substrate. Various substrates, such as saccharides, triglycerides and fatty acids, were tested with log P in the range of −3.24 to 23.7. Triolein was the optimum substrate with the efficient immobilization and assimilation of microorganisms. Using cultivation with commercial salad oil as an inexpensive substitute for triolein, repeated batch degradation of n-tetradecane with the selected immobilized bacterium, Brevibacterium ketoglutamicum ATCC15587, could be continued for over 300 h with only slight loss of activity.
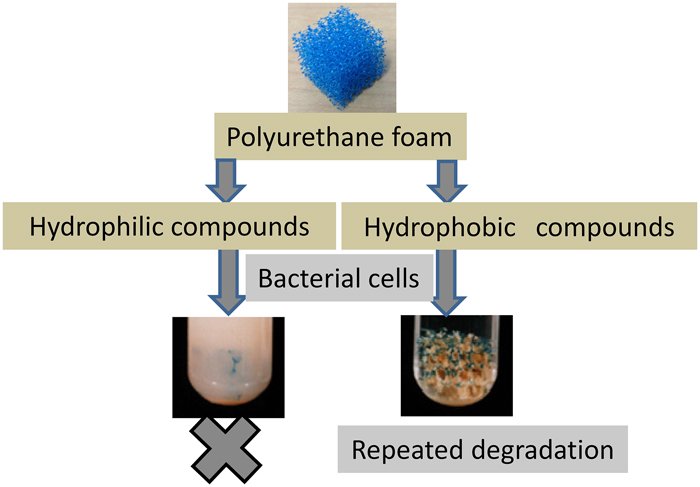 View full abstractDownload PDF (478K)
View full abstractDownload PDF (478K)
- |<
- <
- 1
- >
- >|