- |<
- <
- 1
- >
- >|
-
Article type: Review
2018Volume 64Issue 4 Pages 289-296
Published: 2018
Released on J-STAGE: August 20, 2018
Advance online publication: May 18, 2018Download PDF (1198K)
-
 Article type: Original Article
Article type: Original Article
2018Volume 64Issue 4 Pages 297-301
Published: 2018
Released on J-STAGE: August 20, 2018
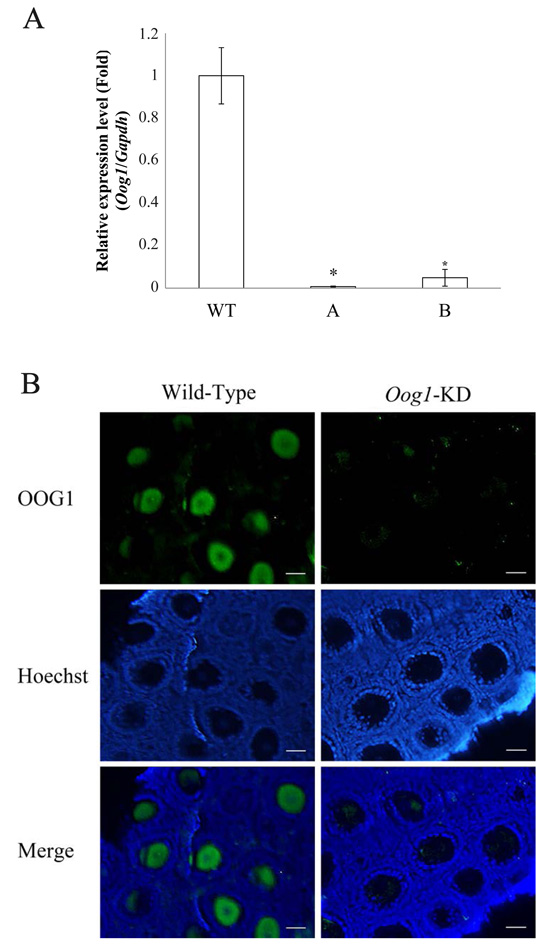
Advance online publication: May 03, 2018Editor's pickCover Story: Oog1, an oocyte-specific gene, encodes the protein belonging to the leucine-rich repeat (LRR) superfamily. LRR is a motif involved in protein-protein interactions. Complete knockout of Oog1 is challenging because five copies of the Oog1 gene are present on chromosomes 4 and 12. Honda et al. generated Oog1 RNA interference (RNAi)-transgenic mice to investigate the effects of Oog1 knockdown on gene expression in the oocytes (Honda et al. Oocyte-specific gene Oog1 suppresses the expression of spermatogenesis-specific genes in oocytes, pp. 297–301). The abundance of spermatogenesis-specific transcripts was elevated in the Oog1 knockdown ovaries. In addition, a few abnormal oocytes were observed in 6-month-old Oog1 knockdown mouse ovaries. These findings suggested that OOG1 suppresses the expression of spermatogenesis-specific genes in the oocytes and plays important roles during oogenesis.
Download PDF (1091K) -
Article type: Original Article
2018Volume 64Issue 4 Pages 303-309
Published: 2018
Released on J-STAGE: August 20, 2018
Advance online publication: May 07, 2018Download PDF (834K) -
Article type: Original Article
2018Volume 64Issue 4 Pages 311-317
Published: 2018
Released on J-STAGE: August 20, 2018
Advance online publication: April 30, 2018Download PDF (898K) -
Article type: Original Article
2018Volume 64Issue 4 Pages 319-326
Published: 2018
Released on J-STAGE: August 20, 2018
Advance online publication: May 05, 2018Download PDF (1590K) -
Article type: Original Article
2018Volume 64Issue 4 Pages 327-335
Published: 2018
Released on J-STAGE: August 20, 2018
Advance online publication: May 24, 2018Download PDF (1784K) -
Article type: Original Article
2018Volume 64Issue 4 Pages 337-342
Published: 2018
Released on J-STAGE: August 20, 2018
Advance online publication: May 31, 2018Download PDF (790K) -
Article type: Original Article
2018Volume 64Issue 4 Pages 343-350
Published: 2018
Released on J-STAGE: August 20, 2018
Advance online publication: June 08, 2018Download PDF (3072K) -
Article type: Original Article
2018Volume 64Issue 4 Pages 351-360
Published: 2018
Released on J-STAGE: August 20, 2018
Advance online publication: June 08, 2018Download PDF (1418K)
-
Article type: Technology Report
2018Volume 64Issue 4 Pages 361-364
Published: 2018
Released on J-STAGE: August 20, 2018
Advance online publication: May 27, 2018Download PDF (592K) -
Article type: Technology Report
2018Volume 64Issue 4 Pages 365-369
Published: 2018
Released on J-STAGE: August 20, 2018
Advance online publication: May 24, 2018Download PDF (667K)
- |<
- <
- 1
- >
- >|