
- Issue 6 Pages 337-
- Issue 5 Pages 291-
- Issue 4 Pages 241-
- Issue 3 Pages 161-
- Issue 2 Pages 79-
- Issue 1 Pages 1-
- |<
- <
- 1
- >
- >|
-
Chiyori SAKAMOTO, Masakatsu FUJINOKI, Masafumi KITAZAWA, Satoshi OBAYA ...Article type: Original Article
2021Volume 67Issue 4 Pages 241-250
Published: 2021
Released on J-STAGE: August 27, 2021
Advance online publication: May 12, 2021JOURNAL OPEN ACCESS
Supplementary materialIn the present study, we investigated the regulatory mechanisms underlying sperm hyperactivation enhanced by 5-hydroxytryptamine (5-HT) in hamsters. First, we examined the types of 5-HT receptors that regulate hyperactivation. Hyperactivation was significantly enhanced by 5-HT2A and 5-HT4 receptor agonists. Moreover, the results of the motility assay revealed that 5-HT2A, 5-HT3, and 5-HT4 receptor agonists significantly decreased the velocity and/or amplitude of sperm. Under 5-HT2 receptor stimulation, hyperactivation was associated with phospholipase C (PLC), inositol 1,4,5-trisphosphate (IP3) receptor, soluble adenylate cyclase (sAC), and protein kinase A (PKA). In contrast, under 5-HT4 receptor stimulation, hyperactivation was associated with transmembrane adenylate cyclase (tmAC), sAC, PKA, and CatSper channels. Accordingly, under the condition that sperm are hyperactivated, 5-HT likely stimulates PLC/IP3 receptor signals via the 5-HT2A receptor and tmAC/PKA/CatSper channel signals via the 5-HT4 receptor. After sAC and PKA are activated by these stimulations, sperm hyperactivation is enhanced.
 View full abstractDownload PDF (1908K)
View full abstractDownload PDF (1908K) -
Fernando LÓPEZ-GATIUS, Irina GARCIA-ISPIERTO, Ronald H.F. HUNTERArticle type: Original Article
2021Volume 67Issue 4 Pages 251-255
Published: 2021
Released on J-STAGE: August 27, 2021
Advance online publication: May 30, 2021JOURNAL OPEN ACCESSThis study sought to establish whether temperature gradients between the cervix, vagina, and rectum at and 7 days post-artificial insemination (AI) were associated with the incidence of pregnancy in lactating dairy cows (Experiment I; n = 90 ovulating cows) and to evaluate temperature gradient dynamics from the time of insemination to 7 days post-AI under heat stress conditions (Experiment II; n = 16 ovulating and 4 non-ovulating cows). In Experiment I, 39 cows (43.3%) became pregnant. The odds ratio for pregnancy was 2.5 for each one-tenth of a degree drop in cervical temperature with reference to the control rectal temperature at the time of AI (P = 0.01), whereas the same decrease in the cervix–rectum temperature differential 7 days post-AI resulted in an odds ratio of 0.44 (P = 0.02). In Experiment II, 5 of the ovulating cows (31.3%) became pregnant. The mean values of the vagina–rectum, vagina–cervix, and cervix–rectum temperature differentials at AI (day 0), 8 h, 24 h, and 7 days post-AI changed significantly from day 0 to day 7 (within-subject effect; P < 0.02) in ovulating cows but not in non-ovulating cows. Temperature differentials on days 0 and 7 were similar between ovulating cows and cows of Experiment I. Overall, our findings support the notion that a temperature differential between the caudal cervical canal and rectum at AI may be an indicator of the likelihood of pregnancy. Possible prospects of confirming estrus at the herd-level are also suggested.
 View full abstractDownload PDF (936K)
View full abstractDownload PDF (936K) -
Yoshiki HIRATA, Yusuke KATSUKURA, Yuka HENMI, Ren OZAWA, Sayaka SHIMAZ ...Article type: Original Article
2021Volume 67Issue 4 Pages 257-264
Published: 2021
Released on J-STAGE: August 27, 2021
Advance online publication: June 28, 2021JOURNAL OPEN ACCESS
Supplementary materialAdvanced maternal age is a risk factor for female infertility, and placental dysfunction is considered one of the causes of pregnancy complications. We investigated the effects of advanced maternal aging on pregnancy outcomes and placental senescence. Female pregnant mice were separated into three groups: young (3 months old), middle (8–9 months old), and aged (11–13 months old). Although the body weights of young and middle dams gradually increased during pregnancy, the body weight of aged dams only increased slightly. The placental weight and resorption rate were significantly higher, and live fetal weights were reduced in a maternal age-dependent manner. Although mRNA expression of senescence regulatory factors (p16 and p21) increased in the spleen of aged dams, mRNA expression of p16 did not change and that of p21 was reduced in the placenta of aged dams. Using a cytokine array of proteins extracted from placental tissues, the expression of various types of senescence-associated secretory phenotype (SASP) factors was decreased in aged dams compared with young and middle dams. The aged maternal placenta showed reduced immune cell accumulation compared with the young placenta. Our present results suggest that models using pregnant mice older than 8 months are more suitable for verifying older human pregnancies. These findings suggest that general cellular senescence programs may not be included in the placenta and that placental functions, including SASP production and immune cell accumulation, gradually decrease in a maternal age-dependent manner, resulting in a higher rate of pregnancy complications.
 View full abstractDownload PDF (1414K)
View full abstractDownload PDF (1414K) -
Shinichi WATANABE, Megumi MIURA, Hiromi MORITA, Moeka NISHI, Shin-ichi ...Article type: Original Article
2021Volume 67Issue 4 Pages 265-272
Published: 2021
Released on J-STAGE: August 27, 2021
Advance online publication: July 09, 2021JOURNAL OPEN ACCESSAdvanced reproductive technologies are being applied for the propagation of squirrel monkeys, to ensure their preservation as a genetic resource and the effective use of their gametes in the future. In the present study, oocytes and spermatozoa were collected from live squirrel monkeys, following which piezo intracytoplasmic sperm injection (ICSI) was performed using these gametes. Follicular development was induced by administering equine chorionic gonadotropin (eCG) containing inhibin antiserum to an immature squirrel monkey female. The unilateral ovary was excised after the administration of human chorionic gonadotropin (hCG), to induce ovulation, following which the larger developed follicular oocytes were collected. Follicular oocytes were prepared for ICSI using sperm from the epididymal tail of a unilateral testis extracted from a mature male. The embryos were continuously incubated in CMRL 1066 medium supplemented with 10% (v/v) fetal bovine serum. Embryo culture was performed with cumulus cells. Two experiments of ICSI carried out with three females resulted in 14 mature oocytes from the 49 cumulus-oocyte complexes collected and five embryos, three of which developed into blastocysts. These blastocysts were vitrified, thawed, and transferred to recipient monkeys, but no pregnancies resulted. In conclusion, the present study is the first to successfully produce ICSI-derived blastocysts from MII oocytes obtained by means of hormone administration (a combination of eCG+inhibin antiserum and hCG) and in vitro maturation in immature squirrel monkeys.
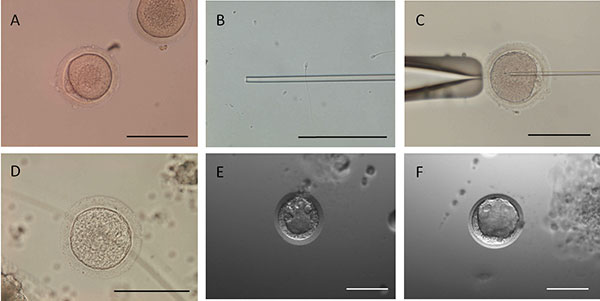 View full abstractDownload PDF (912K)
View full abstractDownload PDF (912K) -
 Riho MORIKAWA, Jibak LEE, Takashi MIYANOArticle type: Original Article
Riho MORIKAWA, Jibak LEE, Takashi MIYANOArticle type: Original Article
2021Volume 67Issue 4 Pages 273-281
Published: 2021
Released on J-STAGE: August 27, 2021
Advance online publication: July 14, 2021JOURNAL OPEN ACCESS
Supplementary materialDuring oocyte growth and follicle development, oocytes closely communicate with cumulus cells. We examined the effects of oocyte-derived growth factors, growth differentiation factor 9 (GDF9) and bone morphogenetic protein 15 (BMP15), on the growth and acquisition of meiotic competence of porcine oocytes collected from early antral follicles (1.2–1.5 mm). First, we confirmed that GDF9 and BMP15 mRNAs were expressed almost exclusively in the oocytes. Oocyte–cumulus cell complexes (OCCs) collected from early antral follicles were cultured in growth medium supplemented with 0–100 ng/ml of GDF9 or BMP15 for 5 days. GDF9 dose-dependently increased the OCC diameter, while BMP15 did not. GDF9 and BMP15 had no significant effects on oocyte growth (P > 0.05). When OCCs that had been cultured with 50 and 100 ng/ml BMP15 were subjected to a subsequent maturation culture, they expanded fully by gonadotropic stimulation and 49% and 61% of oocytes matured to metaphase II (MII), respectively. In contrast, GDF9 did not promote cumulus expansion, and < 10% of oocytes matured to MII. Based on the difference in cumulus expansion, we compared the expression of luteinizing hormone/choriogonadotropin receptor (LHCGR) and follicle stimulating hormone receptor (FSHR) mRNAs in cumulus cells. The level of LHCGR mRNA was increased in cumulus cells of the BMP15 group, although there were no significant differences in FSHR mRNA levels among the groups. These results suggest that GDF9 promotes the growth of OCCs and that BMP15 promotes LHCGR mRNA expression in cumulus cells during oocyte growth culture, which may contribute to cumulus expansion and oocyte maturation.
 View full abstractEditor's pick
View full abstractEditor's pickDuring oocyte growth and follicle development, oocytes closely communicate with cumulus cells. Morikawa et al. examined the effects of oocyte-derived growth factors, growth differentiation factor 9 (GDF9) and bone morphogenetic protein 15 (BMP15), on the growth and acquisition of meiotic competence of porcine oocytes (Morikawa et al. pp. 273–281). Oocyte-cumulus cell complexes (OCCs) collected from early antral follicles were cultured in growth medium supplemented with GDF9 (upper left) and BMP15 (upper right) for 5 days. Only GDF9 increased the OCC diameter in a dose-dependent manner. OCCs that had been cultured with GDF9 or BMP15 were subjected to a subsequent maturation culture. Those OCCs cultured with GDF9 expanded loosely (lower left), whereas those with BMP15 expanded fully and matured to the second metaphase (lower right).
Download PDF (2873K) -
Yuhe TIAN, Qisheng ZHU, Jiayu YUAN, Robert KNEEPKENS, Yuan YUE, Chao Z ...Article type: Original Article
2021Volume 67Issue 4 Pages 283-290
Published: 2021
Released on J-STAGE: August 27, 2021
Advance online publication: July 18, 2021JOURNAL OPEN ACCESSChromium in its trivalent form (chromium (III)) is an essential component of a balanced diet, and its deficiency disturbs glucose and lipid metabolism in humans and animals. The prevailing view is that chromium (III) is notably less toxic than chromium (VI), which is genotoxic and carcinogenic. Thus, the biotransformation of environmental chromium (VI) to chromium (III) is a promising and environmentally friendly detoxification method. However, increasing evidence suggests that chromium (III) induces considerable cytotoxicity. However, the toxicity of chromium (III) to early embryos remains largely unknown. In the present study, we used in vitro fertilization (IVF) to produce mouse embryos and identified the direct embryotoxicity of chromium (III). On exposure to high concentrations of CrCl3, blastocyst formation almost completely failed and a large proportion of embryos were arrested at the 2- to 4-cell stage. At low concentrations of CrCl3, IVF embryos showed a significant decrease in blastocyst formation, reduced total cell numbers, aberrant lineage differentiation, increased oxidative stress, and apoptosis. We also found that chromium (III) exposure during the preimplantation stage, even at low concentrations, led to impaired post-implantation development. Thus, our study substantiates the direct embryotoxicity of chromium (III) during preimplantation development and prolonged impairment of development potential. The results further highlight the potential adverse effects of chromium (III) on public reproductive health with respect to increased environmental enrichment of and dietary supplementation with chromium (III) complexes.
 View full abstractDownload PDF (5062K)
View full abstractDownload PDF (5062K)
- |<
- <
- 1
- >
- >|