- |<
- <
- 1
- >
- >|
-
Article type: Opinions and Hypotheses
2019 Volume 65 Issue 1 Pages 1-5
Published: 2019
Released on J-STAGE: February 08, 2019
Advance online publication: December 06, 2018Download PDF (840K)
-
Article type: Original Article
2019 Volume 65 Issue 1 Pages 7-17
Published: 2019
Released on J-STAGE: February 08, 2019
Advance online publication: October 16, 2018Download PDF (10547K) -
Article type: Original Article
2019 Volume 65 Issue 1 Pages 19-27
Published: 2019
Released on J-STAGE: February 08, 2019
Advance online publication: October 13, 2018Download PDF (1871K) -
Article type: Original Article
2019 Volume 65 Issue 1 Pages 29-36
Published: 2019
Released on J-STAGE: February 08, 2019
Advance online publication: November 04, 2018Download PDF (1970K) -
Article type: Original Article
2019 Volume 65 Issue 1 Pages 37-46
Published: 2019
Released on J-STAGE: February 08, 2019
Advance online publication: November 12, 2018Download PDF (6791K) -
Article type: Original Article
2019 Volume 65 Issue 1 Pages 47-55
Published: 2019
Released on J-STAGE: February 08, 2019
Advance online publication: November 16, 2018Download PDF (2917K) -
 Article type: Original Article
Article type: Original Article
2019 Volume 65 Issue 1 Pages 57-66
Published: 2019
Released on J-STAGE: February 08, 2019
Advance online publication: November 22, 2018Editor's pickCover Story:
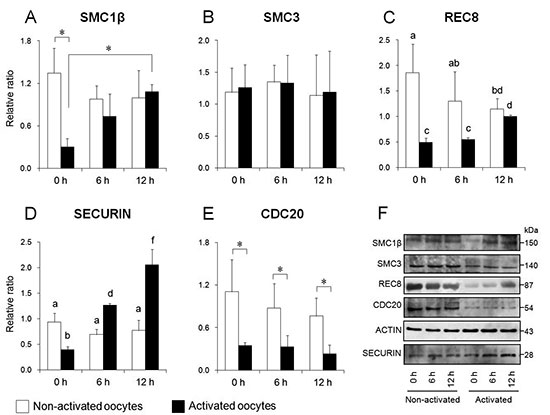
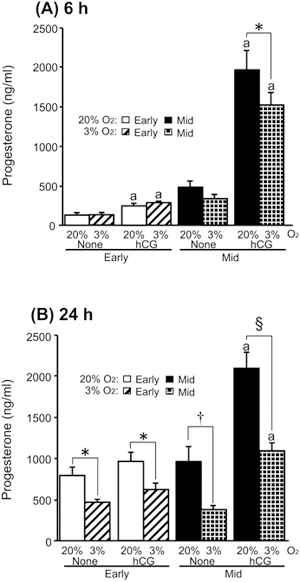
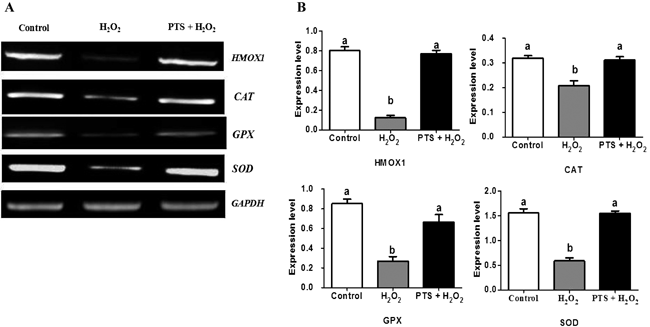
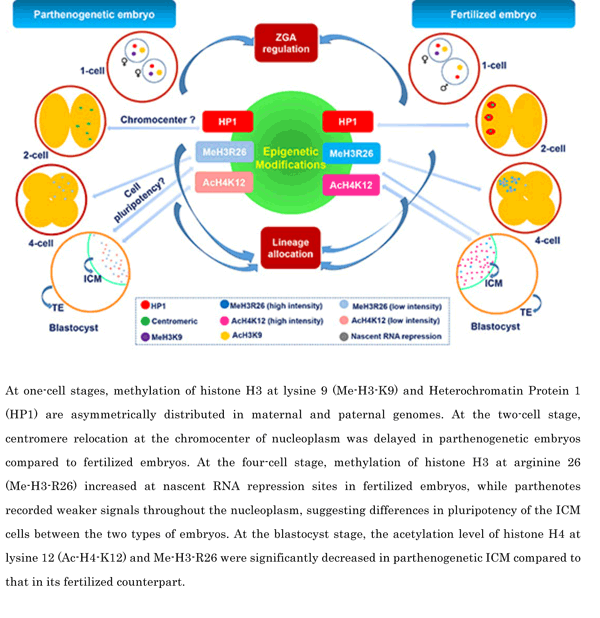
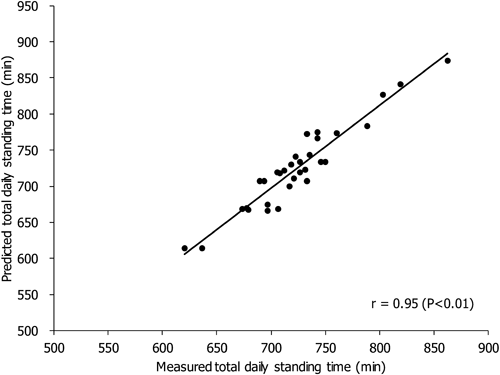
Mammalian oocyte quality degrades over time after in vitro ovulation. As various oocyte manipulations employed in assisted reproductive technology are time consuming, post-ovulatory aging is a serious problem in reproductive medicine and ova research. Shimoi et al. investigated the effects of post-ovulatory aging on the incidence of chromosomal aneuploidy during meiosis II (MII), with a focus on the expression of functional proteins from the spindle assembly checkpoint (SAC) (Shimoi G et al.: Destabilization of spindle assembly checkpoint causes aneuploidy during meiosis II in murine post-ovulatory aged oocytes. pp. 57–66). This study showed that post-ovulatory oocyte aging inhibits MAD2 localization to the sister kinetochore. Furthermore, oocyte aging prevented cohesin subunits from being appropriately maintained or degraded. These results suggest that destabilization of SAC signaling causes sister chromatid segregation errors in MII oocytes and consequently increases the incidence of aneuploidy in early embryos.Download PDF (1887K) -
Article type: Original Article
2019 Volume 65 Issue 1 Pages 67-72
Published: 2019
Released on J-STAGE: February 08, 2019
Advance online publication: November 28, 2018Download PDF (715K) -
Article type: Original Article
2019 Volume 65 Issue 1 Pages 73-81
Published: 2019
Released on J-STAGE: February 08, 2019
Advance online publication: November 15, 2018Download PDF (3986K) -
Article type: Original Article
2019 Volume 65 Issue 1 Pages 83-90
Published: 2019
Released on J-STAGE: February 08, 2019
Advance online publication: December 29, 2018Download PDF (3589K)
-
Article type: Technology Report
2019 Volume 65 Issue 1 Pages 91-95
Published: 2019
Released on J-STAGE: February 08, 2019
Advance online publication: November 03, 2018Download PDF (1030K)
- |<
- <
- 1
- >
- >|