- |<
- <
- 1
- >
- >|
-
Article type: SRD Outstanding Research Award 2022
2023Volume 69Issue 3 Pages 129-138
Published: 2023
Released on J-STAGE: June 06, 2023
Advance online publication: March 16, 2023Download PDF (4494K)
-
 Article type: Review
Article type: Review
2023Volume 69Issue 3 Pages 139-146
Published: 2023
Released on J-STAGE: June 06, 2023
Advance online publication: March 17, 2023Editor's pickCover Story:
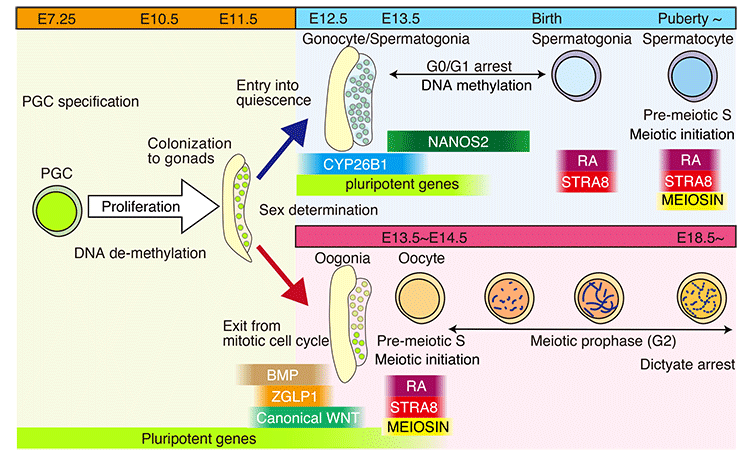
The development of germ cells is accompanied by alterations in the cell cycle in response to external signals and intrinsic cellular mechanisms. During fetal development, male germ cells undergo G0/G1 arrest, whereas female germ cells exit the mitotic phase of the cell cycle and enter meiosis. The NANOS2 and CYP26B1 proteins in the fetal testes cause the germ cells to remain in G0/G1 arrest, which prevents them from entering the meiotic cell cycle. External signals such as RA, BMP, and WNT promote the female germ cells in the fetal ovaries to enter the meiotic phase of the cell cycle. MEIOSIN and STRA8 are transiently co-expressed in the pre-leptotene phase in spermatocytes and oocytes. The MEIOSIN-STRA8 complex ensures the establishment of the meiotic phase by activating meiotic genes in such a manner that the entry into meiosis coincides with the S phase of the cell cycle. This review discusses the development of germ cells from the viewpoint of cell cycle regulation and highlights the mechanism by which germ cells enter the meiotic phase of the cell cycle (Shimada and Ishiguro. Cell cycle regulation for meiosis in mammalian germ cells, pp. 139–146).Download PDF (5886K)
-
Article type: Original Article
2023Volume 69Issue 3 Pages 147-153
Published: 2023
Released on J-STAGE: June 06, 2023
Advance online publication: March 18, 2023Download PDF (1629K) -
Article type: Original Article
2023Volume 69Issue 3 Pages 154-162
Published: 2023
Released on J-STAGE: June 06, 2023
Advance online publication: April 18, 2023Download PDF (2594K) -
Article type: Original Article
2023Volume 69Issue 3 Pages 163-169
Published: 2023
Released on J-STAGE: June 06, 2023
Advance online publication: April 13, 2023Download PDF (1472K) -
Article type: Original Article
2023Volume 69Issue 3 Pages 170-177
Published: 2023
Released on J-STAGE: June 06, 2023
Advance online publication: April 18, 2023Download PDF (5324K) -
Article type: Original Article
2023Volume 69Issue 3 Pages 178-182
Published: 2023
Released on J-STAGE: June 06, 2023
Advance online publication: April 17, 2023Download PDF (1673K)
- |<
- <
- 1
- >
- >|