
- |<
- <
- 1
- >
- >|
-
Hiroki Tanaka, Katsuhiro Sasaki, Shinji TateyamaArticle type: Regular Article
2022Volume 63Issue 9 Pages 1205-1210
Published: September 01, 2022
Released on J-STAGE: August 25, 2022
JOURNAL FREE ACCESS FULL-TEXT HTMLIn this study, precipitation behavior in hot workings on Al–1%Mn alloy was investigated. It has been identified specimens used in this study show thermally stabilized substructures after plain strain compression (PSC) test as a hot working. PSC tests were carried out between 300°C and 500°C. By using small angle x-ray scattering (SAXS) method, dispersion state of precipitations formed in hot workings were estimated. It was considered minute precipitations less than 10 nm in diameter were formed in hot worked areas. These minute precipitations were increased with increasing in compression temperature. In invariant area heated at 400°C, precipitations less than 10 nm were not identified. By using of HAADF-STEM, these minute precipitations less than 10 nm were confirmed, and it was cleared due to EDS analysis the precipitations consist of four elements such as aluminum, manganese, iron and silicon.
This Paper was Originally Published in Japanese in J. JILM 71 (2021) 549–554. The caption of Fig. 6 is slightly modified.
View full abstractDownload PDF (4411K) Full view HTML -
Yicheng Zhang, Koji Inoue, Manabu Ishimaru, Tatsuya Tokunaga, Hidenori ...Article type: Regular Article
2022Volume 63Issue 9 Pages 1211-1216
Published: September 01, 2022
Released on J-STAGE: August 25, 2022
Advance online publication: July 25, 2022JOURNAL FREE ACCESS FULL-TEXT HTMLIn this study, nanoscale phase formation in rapidly solidified Fe20Co20Ni20Cr20B20−xSix alloys was investigated using atom probe tomography (APT) and transmission electron microscopy (TEM). According to previous X-ray diffraction measurements and scanning electron microscopy with energy-dispersive X-ray spectroscopy results, it was thought that this alloy system was composed of almost a single face-centered cubic (FCC) solid solution phase when the Si content was 5 at.%. However, APT and TEM results at the nanoscale showed that both an FCC solid solution and compound phases formed for all alloy compositions. The rapidly solidified alloy ribbon with a Si content of 5 at.% showed the presence of nanoscale FCC and Cr2B phases. Furthermore, in addition to the nanoscale FCC and Cr2B phases, a nanoscale Cr3Ni5Si2 phase was observed when the Si content exceeded 7.5 at.%.
 Projected atom maps of Fe20Co20Ni20Cr20B15Si5 alloy. Fullsize ImageView full abstractDownload PDF (4925K) Full view HTML
Projected atom maps of Fe20Co20Ni20Cr20B15Si5 alloy. Fullsize ImageView full abstractDownload PDF (4925K) Full view HTML
-
O. Olaye, O. A. OjoArticle type: Regular Article
2022Volume 63Issue 9 Pages 1217-1223
Published: September 01, 2022
Released on J-STAGE: August 25, 2022
JOURNAL FREE ACCESS FULL-TEXT HTMLThe newly reported forward simulation method is used to extract concentration-dependent interdiffusion coefficient (D=F(C)) from experimental concentration profiles obtained under constant and time-varying surface concentration conditions, which is impossible by the standard analytical methods. Also, theoretical D=F(C) under constant and time-varying surface concentration conditions are computed in systems with diffusion-induced stress generation and relaxation. The experimental and theoretical results show that the long-held general assumption that D=F(C) is the same for constant surface concentration and time-varying surface concentration is not valid, and such assumption can cause model prediction errors in cases where a surface concentration changes with time. These include the use of D=F(C) computed by the standard analytical techniques, such as the Boltzmann-Matano, Sauer-Freise, Hall, and Wagner methods, for predicting diffusion during homogenization processes.
 Experimental analysis and theoretical analysis based on DIS generation and relaxation show that D=F(C) for systems with constant and time-varying concentration can be different. Fullsize ImageView full abstractDownload PDF (3096K) Full view HTML
Experimental analysis and theoretical analysis based on DIS generation and relaxation show that D=F(C) for systems with constant and time-varying concentration can be different. Fullsize ImageView full abstractDownload PDF (3096K) Full view HTML -
Tomotsugu Shimokawa, Kazuki Hara, Tomoaki NiiyamaArticle type: Regular Article
2022Volume 63Issue 9 Pages 1224-1231
Published: September 01, 2022
Released on J-STAGE: August 25, 2022
Advance online publication: July 25, 2022JOURNAL FREE ACCESS FULL-TEXT HTMLIn order to investigate the synergistic effect of different deformation modes (dislocations and local atomistic rearrangements) on the strength of mixed crystalline and amorphous materials, tensile and compressive deformation analyses of a two-dimensional binary system with various microstructures is performed through molecular dynamics simulations. The binary system is composed of atoms with two different atomic radii. By varying the mixing ratio and the interaction force between different atoms, 66 binary system models with various structures are represented, and each model is classified into three categories, crystalline, amorphous, and mixed crystalline/amorphous, through structural analysis. Deformation analysis shows that the strength of the mixed crystalline/amorphous models tends to be weaker than that of the crystal and amorphous models. The is because the edge of the force chain in the amorphous phase appears at the crystalline/amorphous interface, where dislocation release from the interface to the crystalline phase occurs easily.
This Paper was Originally Published in Japanese in J. Soc. Mater. Sci., Japan 71 (2022) 135–142.
 Fig. 10 Force chain development around the amorphous/crystalline interface shown in Fig. 9 under compressive deformation. Fullsize ImageView full abstractDownload PDF (7852K) Full view HTML
Fig. 10 Force chain development around the amorphous/crystalline interface shown in Fig. 9 under compressive deformation. Fullsize ImageView full abstractDownload PDF (7852K) Full view HTML -
 Yuma Aoki, Motomichi Koyama, Masaki Tanaka, Kaneaki TsuzakiArticle type: Regular Article
Yuma Aoki, Motomichi Koyama, Masaki Tanaka, Kaneaki TsuzakiArticle type: Regular Article
2022Volume 63Issue 9 Pages 1232-1241
Published: September 01, 2022
Released on J-STAGE: August 25, 2022
Advance online publication: August 05, 2022JOURNAL FREE ACCESS FULL-TEXT HTMLWe investigated how dwell fatigue loading accelerates the crack propagation in bi-modal Ti–6Al–4V alloy. A fatigue test was programmed to include displacement holding for only one cycle at several ΔK. We found (1) crack tip strain evolved during the displacement holding, (2) the displacement holding increased fatigue striation spacing, and (3) the strain increment during the displacement holding was linearly correlated with spacing of the displacement-holding-extended striations. These facts indicate that the dwell loading assisted crack opening, which accelerated the crack propagation. Other analyses results and discussion are also presented, in terms of crack propagation mode, crack closure, and dislocation structure.
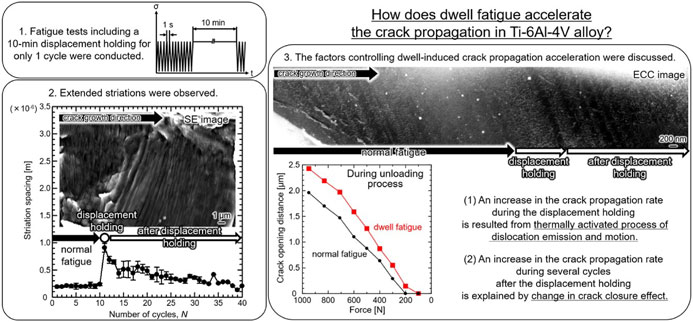 View full abstractEditor's pick
View full abstractEditor's pickBest Paper Award 2023
Download PDF (5151K) Full view HTML
-
Masahiko Hatakeyama, Kenichi Nakano, Ryosuke Yoshita, Daichi Nakato, S ...Article type: Regular Article
2022Volume 63Issue 9 Pages 1242-1247
Published: September 01, 2022
Released on J-STAGE: August 25, 2022
Advance online publication: July 15, 2022JOURNAL FREE ACCESS FULL-TEXT HTMLMagnesium alloys have low corrosion resistance due to their high chemical activity. Therefore, it is necessary to improve their corrosion resistance to use in corrosive environments. In the present study, we aimed to improve the corrosion resistance of AZ91 magnesium alloy in NaCl solution, using aluminum sputtering deposition, anodic oxidation treatment and sealing process were examined. To evaluate the corrosion rate, electrochemical experiments were carried out. In the case of just aluminum sputtering deposition, corrosion resistance was not improved due to small defects in the sputtering deposition. However, corrosion resistance was improved by sputtering deposition followed by interdiffusion treatment at 553 K for more than 3 hours. Corrosion resistance was further improved by anodic oxidation treatment and sealing process after the interdiffusion treatment. With this series of treatments, the corrosion rate of the samples was reduced to 1/360 compared to that of AZ91 base metal. The results show that the interdiffusion treatment, anodic oxidation treatment and sealing process after sputtering deposition are significantly effective to improve corrosion resistance of AZ91 magnesium alloy.
 Fig. 8 Corrosion rate and Corrosion potential of samples measured in 3.5 mass% NaCl solution at 298 K; (a) AZ91 substrate, (b) AZ91 substrate with Al film as-deposited, (c) AZ91 substrate with Al film deposited and interdiffusion treatment at 553 K for 1 hour, (d) for 3 hours, and (e) for 9 hours. (f) AZ91 substrate with Al film deposited and interdiffusion treatment at 553 K for 9 hours, followed by anodized and hot water sealing. In reference data: (g) Al/AZ91D with AAO treatment 15 min.28) Fullsize ImageView full abstractDownload PDF (3759K) Full view HTML
Fig. 8 Corrosion rate and Corrosion potential of samples measured in 3.5 mass% NaCl solution at 298 K; (a) AZ91 substrate, (b) AZ91 substrate with Al film as-deposited, (c) AZ91 substrate with Al film deposited and interdiffusion treatment at 553 K for 1 hour, (d) for 3 hours, and (e) for 9 hours. (f) AZ91 substrate with Al film deposited and interdiffusion treatment at 553 K for 9 hours, followed by anodized and hot water sealing. In reference data: (g) Al/AZ91D with AAO treatment 15 min.28) Fullsize ImageView full abstractDownload PDF (3759K) Full view HTML -
Thai Ha Nguyen, Ram Song, Yohei Harada, Shinji Muraishi, Shinji KumaiArticle type: Regular Article
2022Volume 63Issue 9 Pages 1248-1257
Published: September 01, 2022
Released on J-STAGE: August 25, 2022
JOURNAL FREE ACCESS FULL-TEXT HTMLMn-containing (2 and 4 mass%) Al–Mn-based alloys were fabricated by high-speed twin-roll casting (HSTRC). Al–4 mass%Mn–1 mass%Si strips contained coarse particles in the central band of the cast strips. Microstructure and chemical analyses revealed that the particles were primarily Al6(MnFe) and β-Al(MnFe)Si. These particles formed during the solidification process of the residual liquid, which slowly cooled after the strip exited the roll gap during HSTRC. However, blowing compressed air onto the strip surface as it exited the roll gap rapidly cooled the residual liquid, reducing the number and size of large particles in the central band region. The cold-rolled and annealed sheets fabricated from the strips exhibited a refined and homogenous microstructure. Consequently, both the strength and elongation of the sheets were improved.
 View full abstractDownload PDF (10182K) Full view HTML
View full abstractDownload PDF (10182K) Full view HTML -
Takuma Minoura, Jun Yaokawa, Hiroaki Iwahori, Yuko Aoki, Mina Iwai, Sh ...Article type: Regular Article
2022Volume 63Issue 9 Pages 1258-1265
Published: September 01, 2022
Released on J-STAGE: August 25, 2022
Advance online publication: July 15, 2022JOURNAL FREE ACCESS FULL-TEXT HTMLTo elucidate the segregation behavior of solutes in Al–Si and Al–Cu binary alloys, specimens of several hypo-eutectic Al–Si alloys water-quenched at different stages of solidification or air-cooled from melt to room temperature were prepared and the distributions of Si concentration were analyzed across the primary dendrites. The Si distribution in the dendrites of the specimens water-quenched during primary solidification showed that Si concentration declined from the surface toward the center of the dendrite. In contrast, the specimens water-quenched after eutectic solidification or air-cooled from melt to room temperature showed that Si concentration increased from the surface toward the center of the dendrite. The diffusion distance of Si in the dendrites during the cooling process from the finish of solidification to room temperature was calculated according to Fick’s second law and showed good agreement with the measured value. Therefore, the segregation behavior with high Si concentration in the center of the dendrite was attributed to the diffusion of Si from the center to the surface of the dendrite, resulting in the precipitation on the adjacent Si phase during eutectic solidification.
This Paper was Originally Published in Japanese in J. JFS 93 (2021) 169–175. Reference 6) is deleted and the number of References is moved up from then.
View full abstractDownload PDF (4607K) Full view HTML
-
Reona Miyazaki, Takamasa Hirai, Takehiko HiharaArticle type: Regular Article
2022Volume 63Issue 9 Pages 1266-1272
Published: September 01, 2022
Released on J-STAGE: August 25, 2022
JOURNAL FREE ACCESS FULL-TEXT HTMLThe effects of Ca2+ doping on the ionic conductivity and the stability with a Li of NaI–LiI solid electrolyte were investigated. Evidence of the Ca2+ doping was found in a Rietveld analysis of the synchrotron X-ray diffraction pattern. The conductivity of 9(9NaI·CaI2)·LiI was measured to be 2 × 10−5 S/cm at 60°C, which was higher than the Ca2+-free sample at the same temperature (1 × 10−5 S/cm). The short-circuit of the Li | 9(9NaI·CaI2)·LiI | Li cell was observed after keeping the cell at the open-circuit. The reductive decomposition of the solid electrolyte to LiI and Ca (or Li–Ca alloy) was suggested by contacting with Li. The short-circuit was attributed to the continuous growth of the Li–Ca alloy. The phase separation at Li/9NaI·LiI interphase was not observed. These results suggest that while Li+ conductors stabilized with Li can be developed based on Na compounds, Ca2+ doping is not an effective strategy to enhance conductivity.
 View full abstractDownload PDF (2752K) Full view HTML
View full abstractDownload PDF (2752K) Full view HTML -
Seiyu Teruya, Noritaka Saito, Kunihiko NakashimaArticle type: Regular Article
2022Volume 63Issue 9 Pages 1273-1280
Published: September 01, 2022
Released on J-STAGE: August 25, 2022
Advance online publication: August 05, 2022JOURNAL FREE ACCESS FULL-TEXT HTMLFine Ni particles are utilized as internal electrode materials for multilayer ceramic capacitors. Despite extensive research, the effects of the surface properties (additional elements) remain unclear. To the best of our knowledge, the present study is the first to demonstrate the effects of the addition of sulfur (S) and phosphorus (P) on surface modification and binder decomposition during the production of fine Ni particles. Scanning electron microscopy, X-ray diffractometry, thermogravimetry-differential thermal analysis, hydrogen temperature-programmed reduction, temperature-programmed decomposition, and X-ray photoelectron spectroscopy were performed to analyze non-doped Ni, S-doped Ni and P-doped Ni powder. S existed as S–O or Ni–S on the particle surface in the S-doped Ni powder, whereas P existed solely as P–O on the particle surface in the P-doped Ni powder. When formed into a paste with these powders, the decomposition temperature of the binder is lower than that of the binder alone owing to the catalytic effect of the unmodified surface of Ni in non-doped Ni. However, the catalyst poisoning effect of S in S-doped Ni and the coating effect of P–O in P-doped Ni suppressed the lowering of the binder decomposition temperature due to the catalysis of the Ni surface. The results of the present study are expected to inspire future research on the optimization of the manufacturing process of multilayer ceramic capacitors.
 View full abstractDownload PDF (3516K) Full view HTML
View full abstractDownload PDF (3516K) Full view HTML -
Subaru Tsujimura, Masahiro InoueArticle type: Regular Article
2022Volume 63Issue 9 Pages 1281-1286
Published: September 01, 2022
Released on J-STAGE: August 25, 2022
JOURNAL FREE ACCESS FULL-TEXT HTMLThe effects of binder chemistry on electrical-conductivity development, i.e., dynamic percolation, in carbon nanotube (CNT)-filled epoxy-based pastes during curing were investigated. The electrical resistivities of the pastes are influenced not only by the dispersivity of the CNT filler but also by the binder chemistry of the pastes. The electrical-conductivity-development kinetics are accelerated by introducing a reactive diluent, namely phenyl glycidyl ether, to the binder. As shown by alternating current impedance spectroscopy, the electrical-conductivity development is governed by the decrease in interfacial electrical resistance between the filler units. Electrical-conductivity development does not occur synchronously with the curing shrinkage of the binder; thus, the chemical state of the interface adjacent to the fillers is a key parameter for enhancing electrical conductivity. Thus, control of this interfacial chemistry is an essential concept for developing electrically conductive CNT-filled pastes.
 Effect of binder chemistry on electrical conductivity-development (dynamic percolation) in carbon-nanotube-filled pastes during curing. Fullsize ImageView full abstractDownload PDF (2520K) Full view HTML
Effect of binder chemistry on electrical conductivity-development (dynamic percolation) in carbon-nanotube-filled pastes during curing. Fullsize ImageView full abstractDownload PDF (2520K) Full view HTML
-
Ken Adachi, Takumi Anezaki, Tomoro Karube, Atsushi Iizuka, Etsuro Shib ...Article type: Regular Article
2022Volume 63Issue 9 Pages 1287-1293
Published: September 01, 2022
Released on J-STAGE: August 25, 2022
Advance online publication: July 08, 2022JOURNAL FREE ACCESS FULL-TEXT HTMLArsenic-containing wastewater, which is mainly generated in nonferrous metal-smelting plants, threatens the natural environment and human health owing to its high toxicity and mobility. A promising arsenic immobilization method to solve this problem is to immobilize arsenic as scorodite. In this study, the method of scorodite synthesis using magnetite as the iron source was investigated, which can be performed using low-cost materials under atmospheric pressure without using an autoclave. The generation and crystallization behaviors of the intermediate of the gel-like precursor of scorodite were investigated by monitoring the concentration of each iron ion in solution and characterizing the precipitate. By clarifying the effect of the solution pH for each reaction step, the mechanism and suitable conditions of this process are discussed.
View full abstractDownload PDF (5756K) Full view HTML
- |<
- <
- 1
- >
- >|