Volume 79, Issue 2
Displaying 1-42 of 42 articles from this issue
- |<
- <
- 1
- >
- >|
-
2011Volume 79Issue 2 Pages 1-12
Published: 2011
Released on J-STAGE: June 07, 2013
Download PDF (3849K)
-
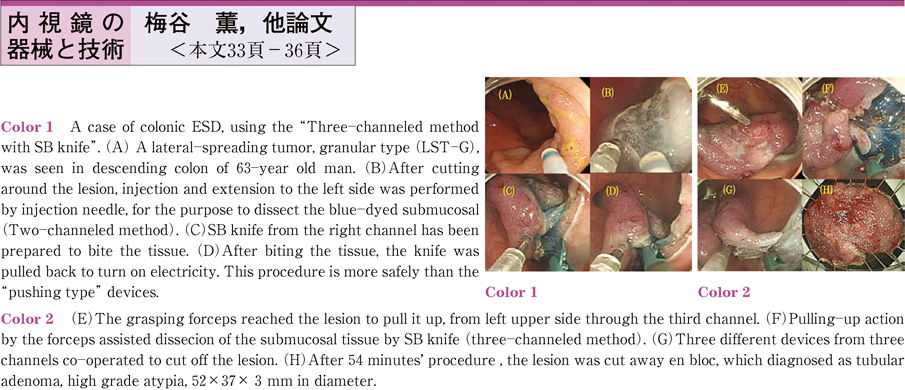
2011Volume 79Issue 2 Pages 33-36
Published: December 10, 2011
Released on J-STAGE: June 07, 2013
Download PDF (778K) -
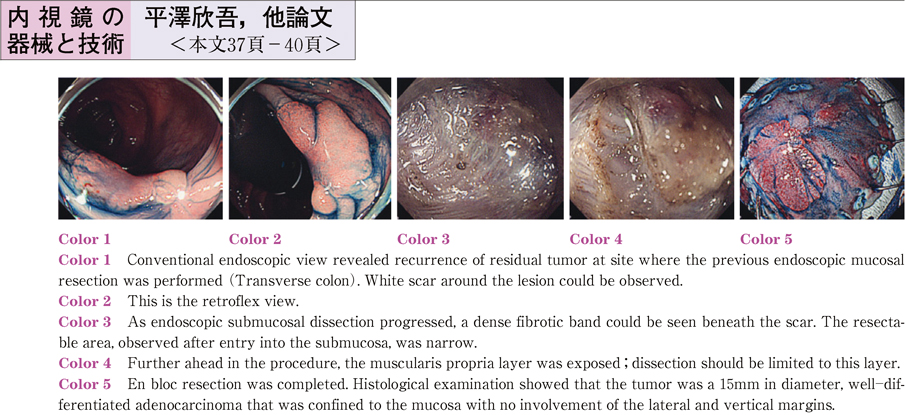
2011Volume 79Issue 2 Pages 37-40
Published: December 10, 2011
Released on J-STAGE: June 07, 2013
Download PDF (676K)
-
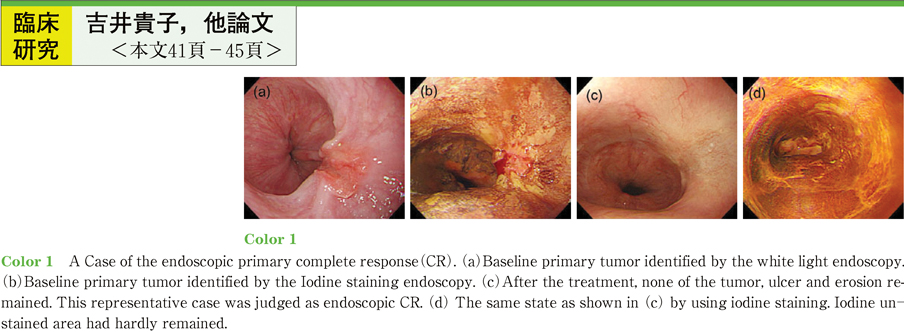
2011Volume 79Issue 2 Pages 41-45
Published: December 10, 2011
Released on J-STAGE: June 07, 2013
Download PDF (766K) -
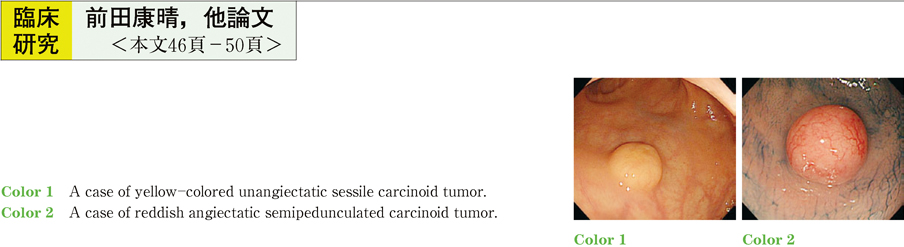
2011Volume 79Issue 2 Pages 46-50
Published: December 10, 2011
Released on J-STAGE: June 07, 2013
Download PDF (788K)
-
2011Volume 79Issue 2 Pages 52-53
Published: December 10, 2011
Released on J-STAGE: June 07, 2013
Download PDF (687K) -
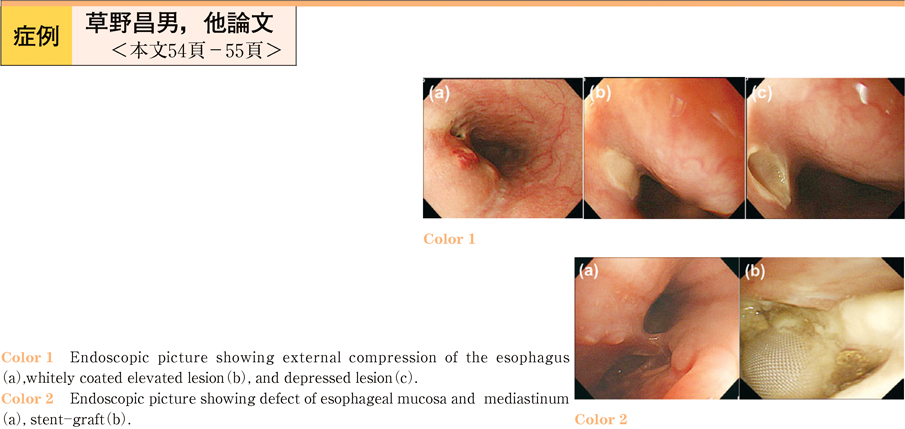
2011Volume 79Issue 2 Pages 54-55
Published: December 10, 2011
Released on J-STAGE: June 07, 2013
Download PDF (763K) -
2011Volume 79Issue 2 Pages 56-57
Published: December 10, 2011
Released on J-STAGE: June 07, 2013
Download PDF (726K) -
2011Volume 79Issue 2 Pages 58-59
Published: December 10, 2011
Released on J-STAGE: June 07, 2013
Download PDF (662K) -
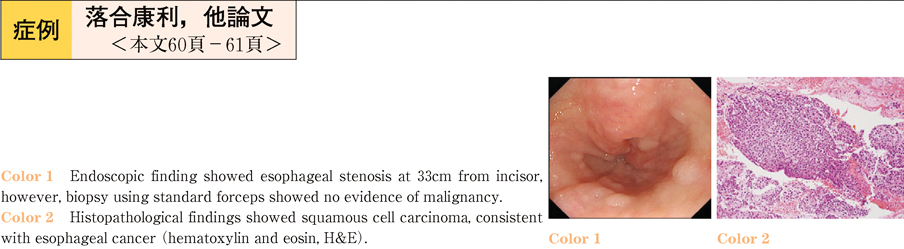
2011Volume 79Issue 2 Pages 60-61
Published: December 10, 2011
Released on J-STAGE: June 07, 2013
Download PDF (705K) -
2011Volume 79Issue 2 Pages 62-63
Published: December 10, 2011
Released on J-STAGE: June 07, 2013
Download PDF (636K) -
A case of stomach ulcer bleeding due to a splenic artery pseudo-aneurysm formed in ulceration bottom2011Volume 79Issue 2 Pages 64-65
Published: December 10, 2011
Released on J-STAGE: June 07, 2013
Download PDF (765K) -
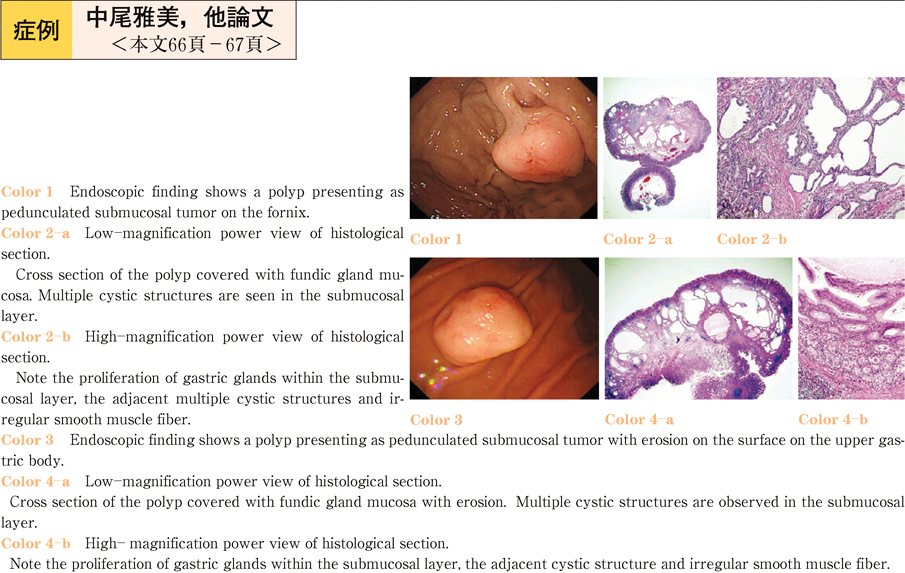
Two cases of hamartomatous inverted polyp of the stomach presenting as pedunculated submucosal tumor2011Volume 79Issue 2 Pages 66-67
Published: December 10, 2011
Released on J-STAGE: June 07, 2013
Download PDF (713K) -
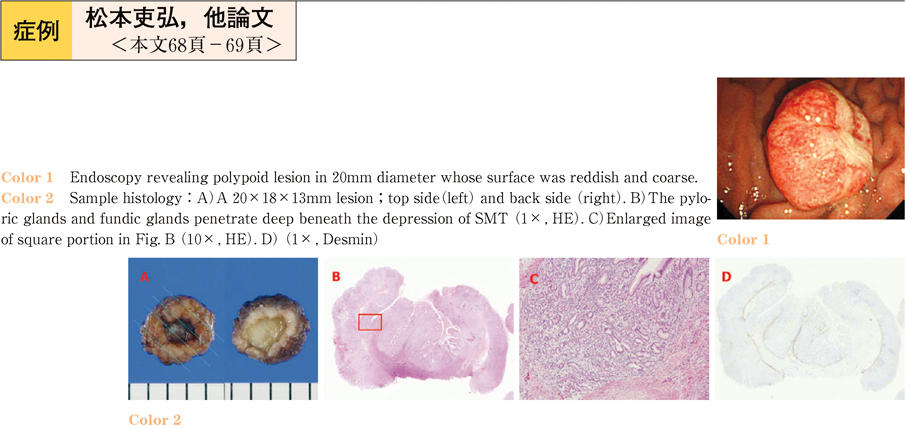
2011Volume 79Issue 2 Pages 68-69
Published: December 10, 2011
Released on J-STAGE: June 07, 2013
Download PDF (632K) -
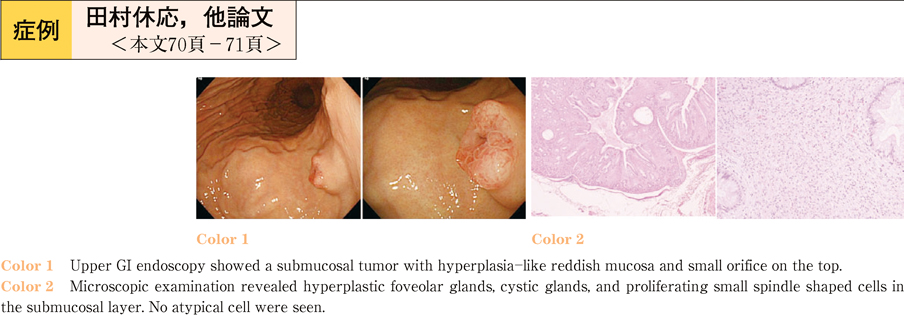
2011Volume 79Issue 2 Pages 70-71
Published: December 10, 2011
Released on J-STAGE: June 07, 2013
Download PDF (724K) -
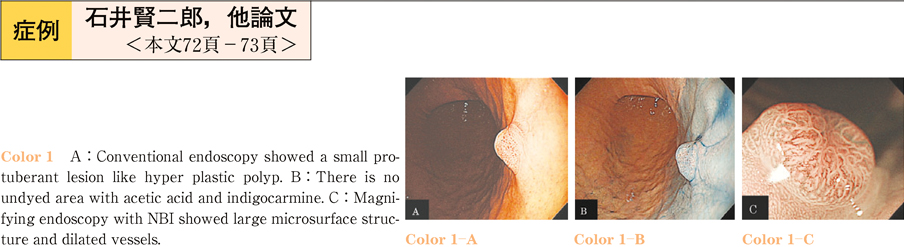
2011Volume 79Issue 2 Pages 72-73
Published: December 10, 2011
Released on J-STAGE: June 07, 2013
Download PDF (741K) -
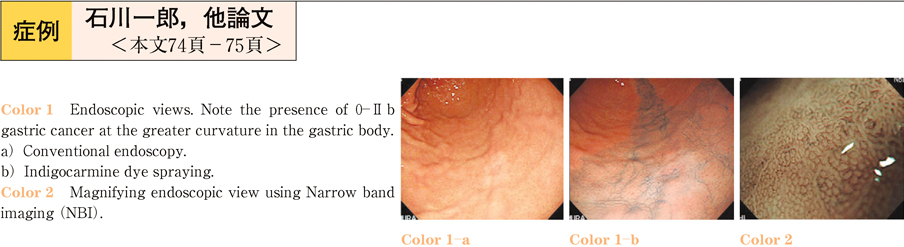
2011Volume 79Issue 2 Pages 74-75
Published: December 10, 2011
Released on J-STAGE: June 07, 2013
Download PDF (706K) -
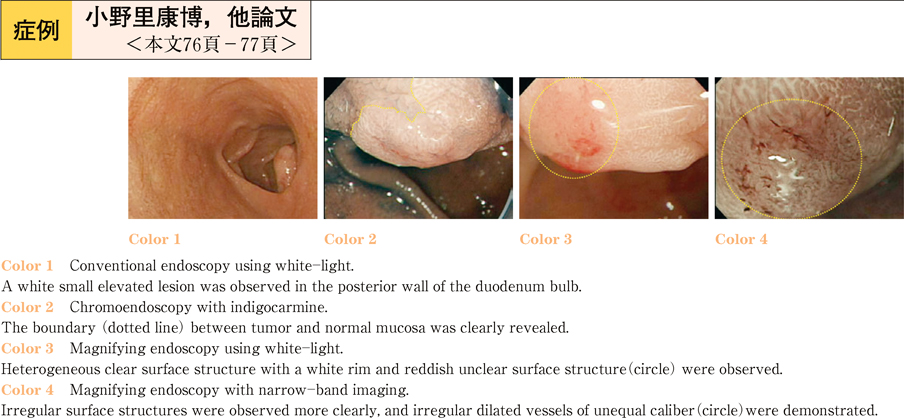
2011Volume 79Issue 2 Pages 76-77
Published: December 10, 2011
Released on J-STAGE: June 07, 2013
Download PDF (688K) -
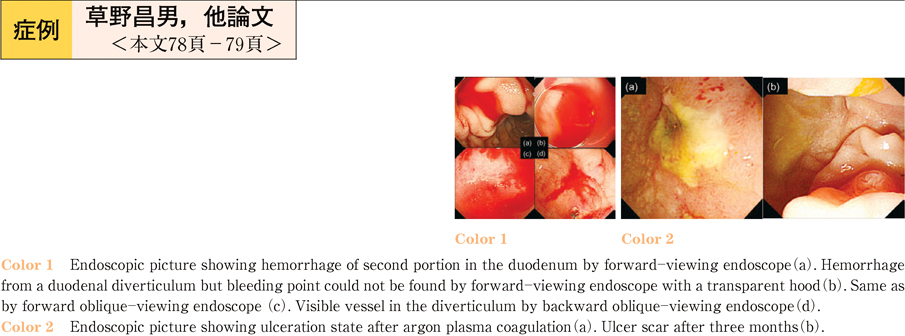
2011Volume 79Issue 2 Pages 78-79
Published: December 10, 2011
Released on J-STAGE: June 07, 2013
Download PDF (790K) -
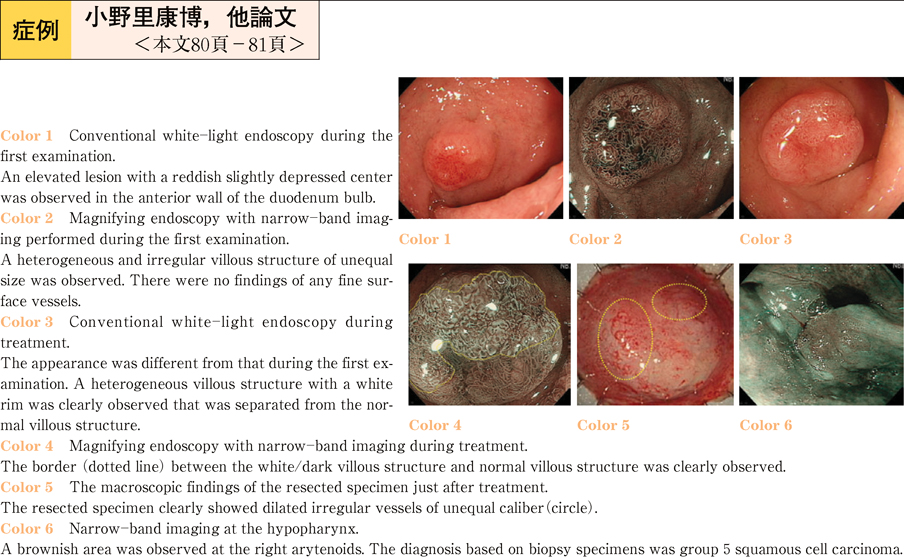
2011Volume 79Issue 2 Pages 80-81
Published: December 10, 2011
Released on J-STAGE: June 07, 2013
Download PDF (680K) -
2011Volume 79Issue 2 Pages 82-83
Published: December 10, 2011
Released on J-STAGE: June 07, 2013
Download PDF (704K) -
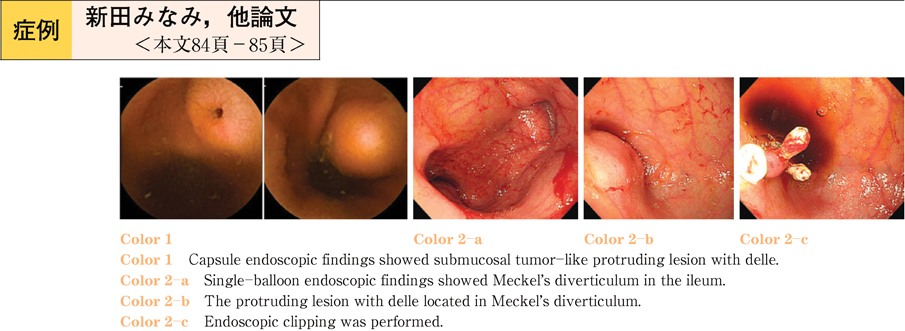
2011Volume 79Issue 2 Pages 84-85
Published: December 10, 2011
Released on J-STAGE: June 07, 2013
Download PDF (704K) -
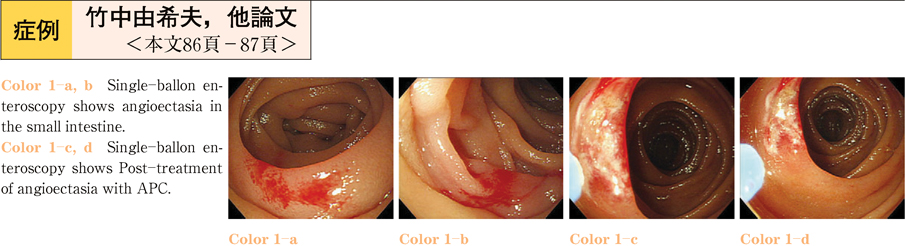
2011Volume 79Issue 2 Pages 86-87
Published: December 10, 2011
Released on J-STAGE: June 07, 2013
Download PDF (660K) -
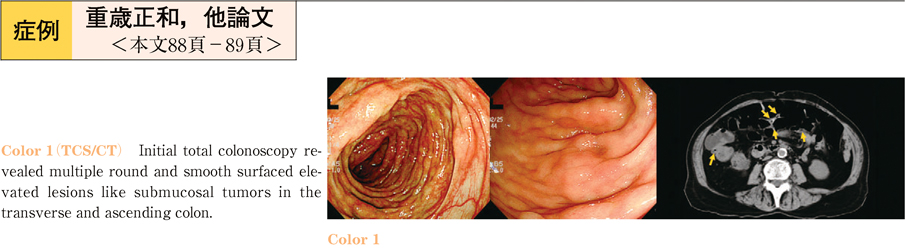
2011Volume 79Issue 2 Pages 88-89
Published: December 10, 2011
Released on J-STAGE: June 07, 2013
Download PDF (605K) -
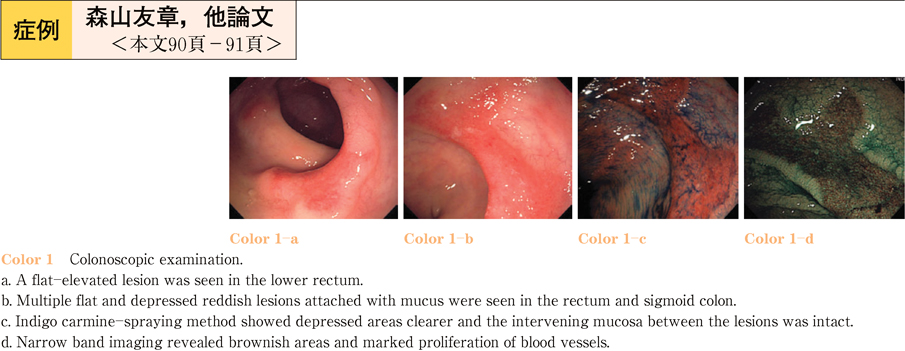
2011Volume 79Issue 2 Pages 90-91
Published: December 10, 2011
Released on J-STAGE: June 07, 2013
Download PDF (767K) -
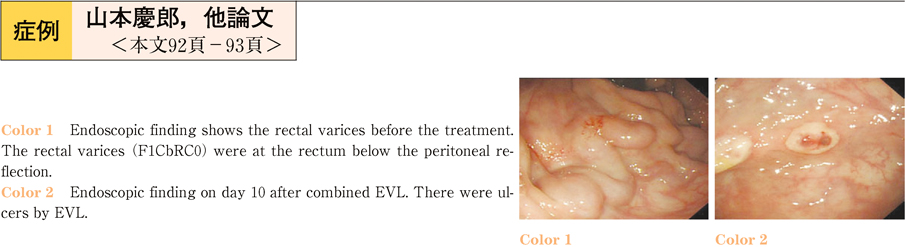
2011Volume 79Issue 2 Pages 92-93
Published: December 10, 2011
Released on J-STAGE: June 07, 2013
Download PDF (707K) -
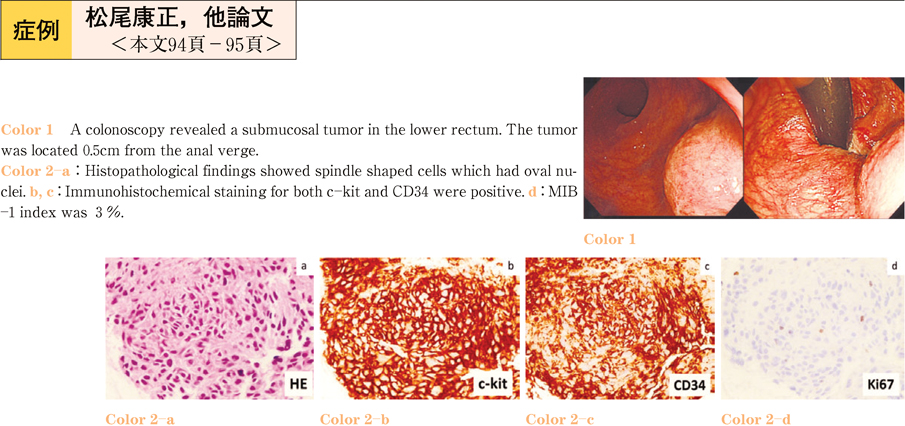
2011Volume 79Issue 2 Pages 94-95
Published: December 10, 2011
Released on J-STAGE: June 07, 2013
Download PDF (754K) -
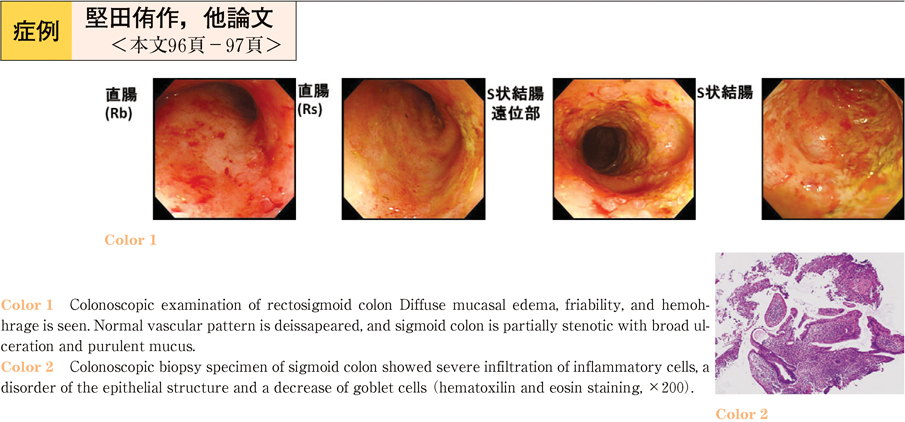
2011Volume 79Issue 2 Pages 96-97
Published: December 10, 2011
Released on J-STAGE: June 07, 2013
Download PDF (745K) -
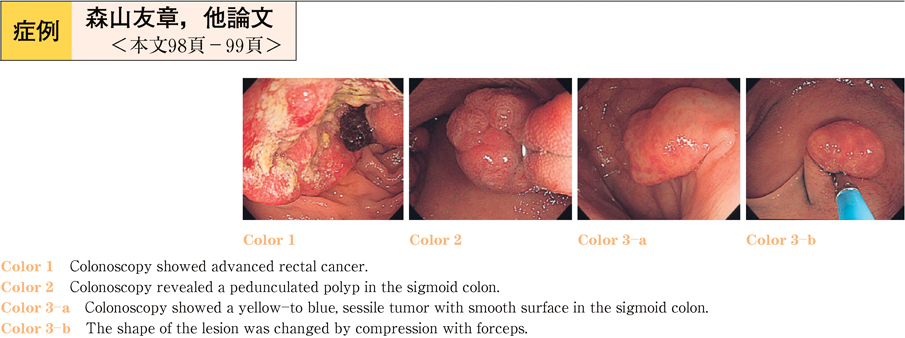
2011Volume 79Issue 2 Pages 98-99
Published: December 10, 2011
Released on J-STAGE: June 07, 2013
Download PDF (735K) -
2011Volume 79Issue 2 Pages 100-101
Published: December 10, 2011
Released on J-STAGE: June 07, 2013
Download PDF (671K) -
2011Volume 79Issue 2 Pages 102-103
Published: December 10, 2011
Released on J-STAGE: June 07, 2013
Download PDF (715K) -
2011Volume 79Issue 2 Pages 104-105
Published: December 10, 2011
Released on J-STAGE: June 07, 2013
Download PDF (757K) -
2011Volume 79Issue 2 Pages 106-107
Published: December 10, 2011
Released on J-STAGE: June 07, 2013
Download PDF (663K) -
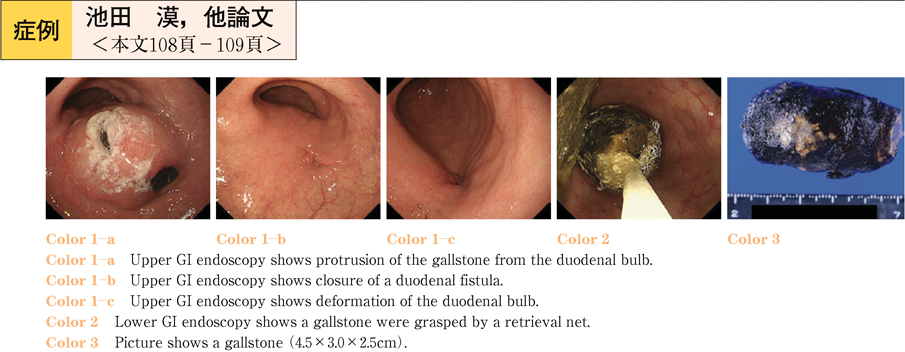
2011Volume 79Issue 2 Pages 108-109
Published: December 10, 2011
Released on J-STAGE: June 07, 2013
Download PDF (725K) -
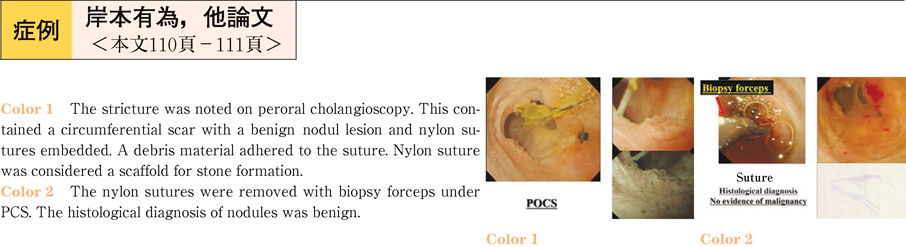
2011Volume 79Issue 2 Pages 110-111
Published: December 10, 2011
Released on J-STAGE: June 07, 2013
Download PDF (659K) -
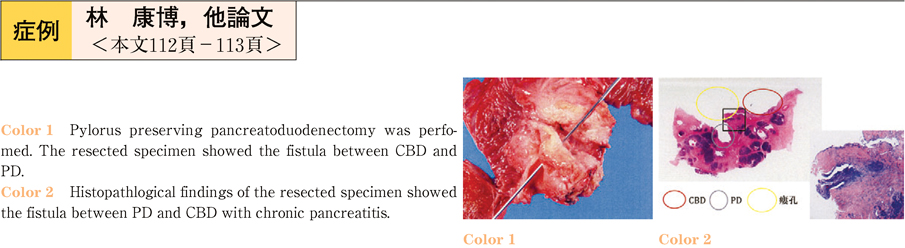
2011Volume 79Issue 2 Pages 112-113
Published: December 10, 2011
Released on J-STAGE: June 07, 2013
Download PDF (796K) -
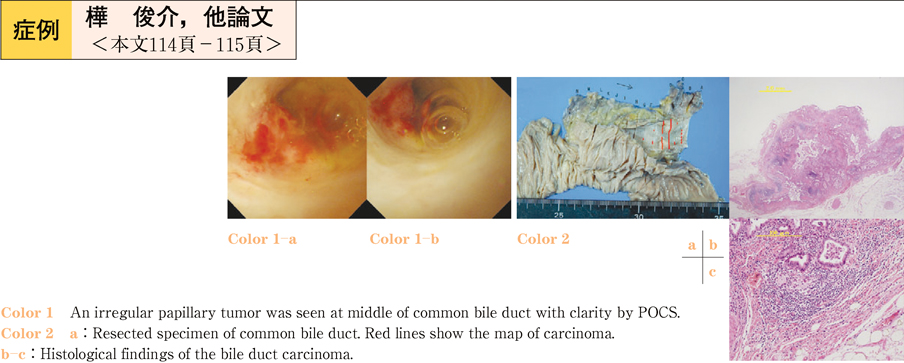
2011Volume 79Issue 2 Pages 114-115
Published: December 10, 2011
Released on J-STAGE: June 07, 2013
Download PDF (812K) -
2011Volume 79Issue 2 Pages 116-117
Published: December 10, 2011
Released on J-STAGE: June 07, 2013
Download PDF (692K) -
2011Volume 79Issue 2 Pages 118-119
Published: December 10, 2011
Released on J-STAGE: June 07, 2013
Download PDF (711K) -
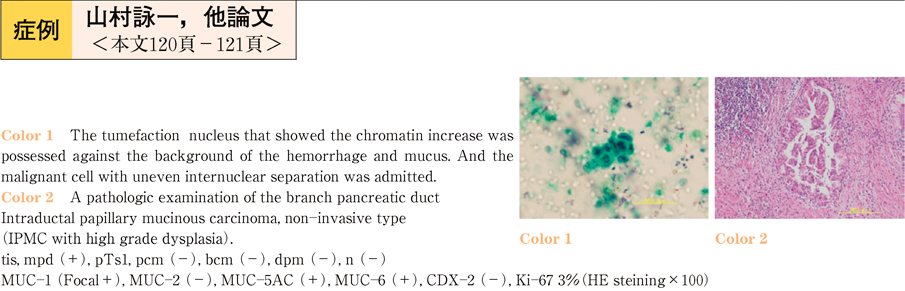
2011Volume 79Issue 2 Pages 120-121
Published: December 10, 2011
Released on J-STAGE: June 07, 2013
Download PDF (669K) -
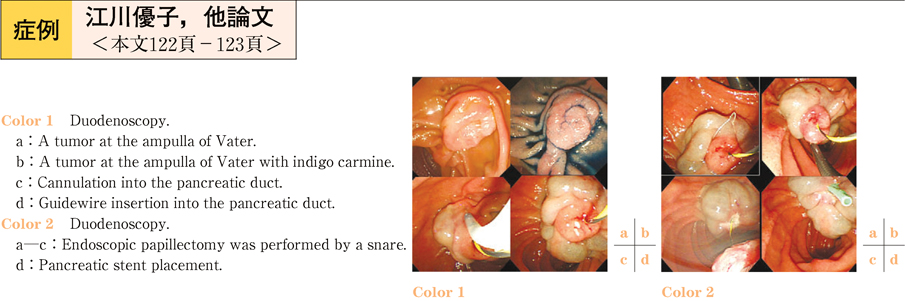
2011Volume 79Issue 2 Pages 122-123
Published: December 10, 2011
Released on J-STAGE: June 07, 2013
Download PDF (674K) -
2011Volume 79Issue 2 Pages 124-125
Published: December 10, 2011
Released on J-STAGE: June 07, 2013
Download PDF (679K)
- |<
- <
- 1
- >
- >|