
- |<
- <
- 1
- >
- >|
-
Yuta MAEYOSHI, Kazuki YOSHII, Hikari SAKAEBE2022 年 90 巻 4 号 p. 047001
発行日: 2022/04/05
公開日: 2022/04/05
[早期公開] 公開日: 2022/03/02ジャーナル オープンアクセス HTML
J-STAGE DataLi metal is the ultimate anode material for rechargeable Li batteries because of its high capacity and low electrochemical potential. However, Li metal anodes suffer from low Coulombic efficiency and poor cycling stability owing to the growth of Li dendrites. In this study, we report that a localized high-concentration electrolyte comprising lithium bis(fluorosulfonyl)imide (LiFSI), ethylene carbonate (EC), propylene carbonate (PC), and 1,1,2,2-tetrafluoroethyl 2,2,3,3-tetrafluoropropyl ether (HFE) achieves stable Li plating/stripping cycling with a Coulombic efficiency of >98 %. In contrast to LiFSI/EC : PC electrolytes, this electrolyte shows good wettability on a polypropylene separator. Li metal deposited in this electrolyte displays a large, granular, and dense morphology. Spectroscopic analyses confirm strong FSI−–Li+ coordination in this electrolyte, leading to the formation of a solid electrolyte interphase (SEI) layer enriched with LiF and sulfurous compounds derived from FSI−. These results indicate that the SEI layer facilitates the deposition of compact Li and effectively prevents Li loss owing to electrolyte decomposition and dead Li formation, resulting in highly reversible Li plating/stripping cycling. This electrolyte design can be an effective strategy for developing high-energy-density Li metal batteries.
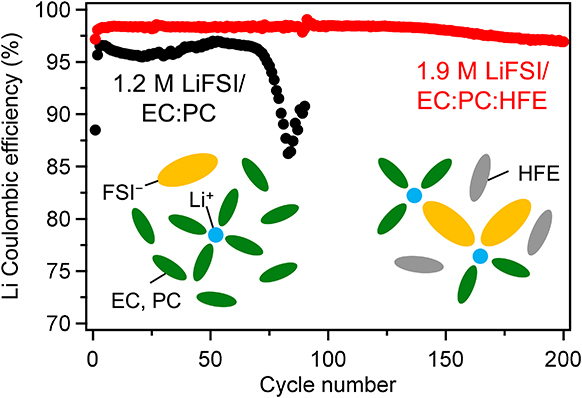 抄録全体を表示PDF形式でダウンロード (2924K) HTML形式で全画面表示
抄録全体を表示PDF形式でダウンロード (2924K) HTML形式で全画面表示 -
Shunsuke KIMURA, Hikaru WAKATSUKI, Kazuki NAKAMURA, Norihisa KOBAYASHI2022 年 90 巻 4 号 p. 047002
発行日: 2022/04/09
公開日: 2022/04/09
[早期公開] 公開日: 2022/03/05ジャーナル オープンアクセス HTML
J-STAGE DataSilver deposition-based electrochromic (EC) device enabled various optical states by using either the electrodeposition of silver thin films or silver nanoparticles on an indium tin oxide electrode. These optical states, including chromatic colors such as cyan, magenta, yellow, and green, are based on absorption by the localized surface plasmon resonance of silver nanoparticles. However, an ideal counter-reaction material that does not affect the color representation by silver nanoparticles and does not dissolve the deposited silver has not been reported. To achieve both color retention and chromatic color representation, MnO2 was utilized as the counter electrode material. The EC properties of the novel device with the MnO2 counter electrode were investigated in terms of charge density and its ability to change color. The novel EC device achieved both representations of chromatic color and retained its color without power supply.
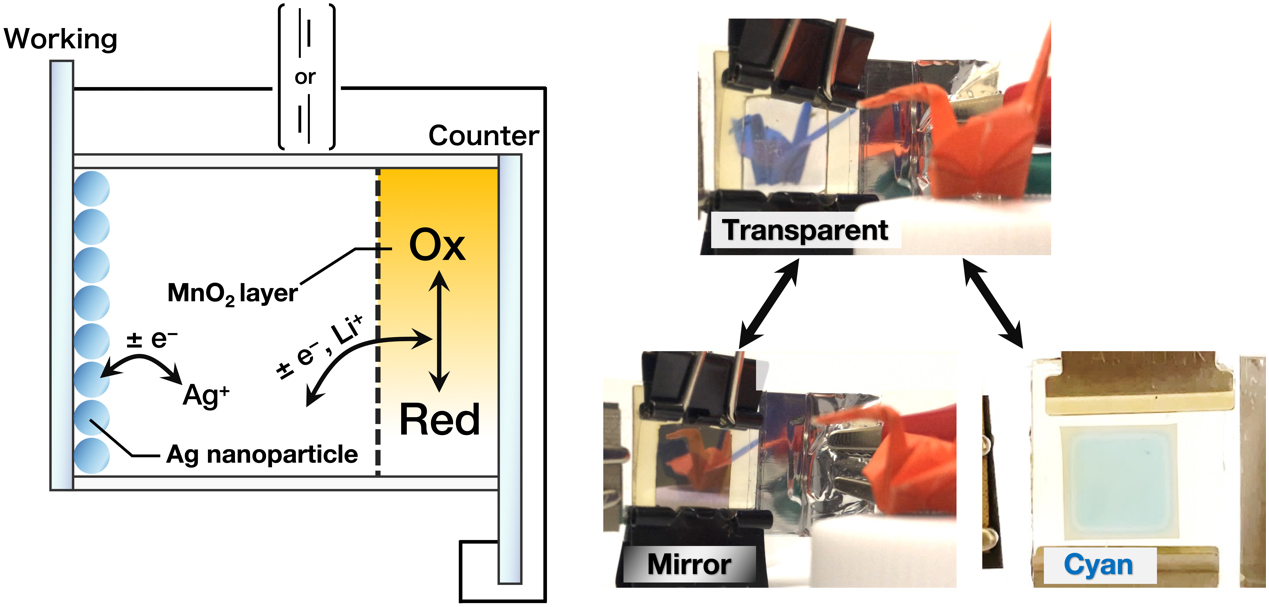 抄録全体を表示PDF形式でダウンロード (3584K) HTML形式で全画面表示
抄録全体を表示PDF形式でダウンロード (3584K) HTML形式で全画面表示 -
Yusuke MORINO, Hirofumi TSUKASAKI, Shigeo MORI2022 年 90 巻 4 号 p. 047003
発行日: 2022/04/21
公開日: 2022/04/21
[早期公開] 公開日: 2022/03/16ジャーナル オープンアクセス HTMLAll-solid-state batteries (ASSBs) using sulfide-based solid electrolytes (SEs) are promising energy storage devices beyond the present liquid-type lithium-ion batteries (LIBs) using organic solvents, which are expected to realize the adaptation of new systems such as higher-voltage cathodes. However, in recent years, undesirable side reactions are being reported in the nanometer-order region at the interface of cathode active materials/SEs. Therefore, we evaluated the cycle durability of the all-solid-state cathode half-cells using an argyrodite-structured sulfide-based solid electrolyte at various potentials at 60 °C and then measured the coulombic behavior by high precision coulometry. Furthermore, the interfaces of the cathode active material/SE were observed using secondary electron microscopy (SEM), transmission electron spectroscopy (TEM), and electron diffraction (ED). In conclusion, a strong correlation was found between the coulombic behavior and material decomposition at the interface.
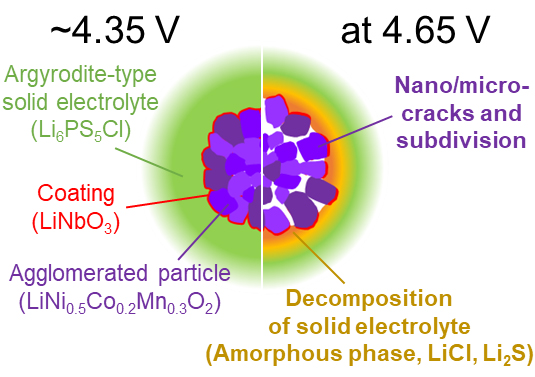 抄録全体を表示PDF形式でダウンロード (5138K) HTML形式で全画面表示
抄録全体を表示PDF形式でダウンロード (5138K) HTML形式で全画面表示 -
Kensaku NAGASAWA, Li KUNPENG, Yu TAKENAGA, Yoshiyuki KURODA, Shigenori ...2022 年 90 巻 4 号 p. 047004
発行日: 2022/04/21
公開日: 2022/04/21
[早期公開] 公開日: 2022/04/02ジャーナル オープンアクセス HTML
J-STAGE DataIn this study, we propose a highly accurate catalyst evaluation method by improving the time-zero analysis, which has been reported as an activity evaluation method for gas evolution powder catalysts. We refined the extrapolation approach to plot in this study’s time-zero analysis as an analytical method. The Tafel slopes obtained with this method for LaNiO3 (LNO) were found to be within the reported range, and the data was consistent in catalytic loading range of 2.5–3.3 mg-LNO cm−2. Using the improved time-zero analysis, the kinetic activity of the gas evolution powder catalyst is possible to be evaluated with high accuracy.
 抄録全体を表示PDF形式でダウンロード (2596K) HTML形式で全画面表示
抄録全体を表示PDF形式でダウンロード (2596K) HTML形式で全画面表示 -
Takuma TAKAYANAGI, Akira NASU, Fumika TSUJI, Atsushi SAKUDA, Masahiro ...2022 年 90 巻 4 号 p. 047005
発行日: 2022/04/21
公開日: 2022/04/21
[早期公開] 公開日: 2022/03/03ジャーナル オープンアクセス HTML
J-STAGE DataSolid electrolytes with high ionic conductivity, high formability, and high electrochemical properties are required to improve the performance of all-solid-state sodium batteries. In this study, we focus on a combination of Na2.88Sb0.88W0.12S4 and NaI for preparing composite electrolytes Na2.88Sb0.88W0.12S4·xNaI, and investigate their crystal structures, microstructures, and electrochemical properties. NaI is uniformly dispersed in the composites without forming a solid solution with Na2.88Sb0.88W0.12S4. Na2.88Sb0.88W0.12S4·0.50NaI shows a high ionic conductivity of 3.6 × 10−2 S cm−1 after sintering at 275 °C for only 1.5 h. The charge-discharge characteristics of all-solid-state cells using the Na2.88Sb0.88W0.12S4·xNaI composite are also improved.
抄録全体を表示PDF形式でダウンロード (1855K) HTML形式で全画面表示
- |<
- <
- 1
- >
- >|