
- |<
- <
- 1
- >
- >|
-
Shingo OKUBO, Yoshihisa OZEKI, Tetsuya YAMADA, Kosuke SAITO, Noboru IS ...2022 年 90 巻 7 号 p. 077001
発行日: 2022/07/06
公開日: 2022/07/06
[早期公開] 公開日: 2022/05/31ジャーナル オープンアクセス HTML
J-STAGE DataIn this study, we propose a facile fabrication process for all-solid-state ion-selective electrodes by laminating and drop-casting; these electrodes are suitable for applications in the environmental, agricultural, and medical fields, such as oral condition monitoring. To date, research has focused only on developing ion-selective electrodes for specific applications. However, an ion-selective electrode with compatibility for wide use has not been realized because specialized processing techniques and equipment are required. Our fabrication process achieved an ion-selective multi-sensor with a wireless system, which is crucial for overcoming the aforementioned challenge. The developed sensors exhibited sufficient sensitivity, repeatability, response time, and selectivity for medical, environmental, and agricultural applications.
抄録全体を表示PDF形式でダウンロード (3201K) HTML形式で全画面表示 -
Jianke LI, Xincheng MIAO, Shenhao WANG, Shaobei CHEN, Beibei HAN, Guiy ...2022 年 90 巻 7 号 p. 077002
発行日: 2022/07/06
公開日: 2022/07/06
[早期公開] 公開日: 2022/06/04ジャーナル オープンアクセス HTML
J-STAGE DataThe coal tar pitch based V2O3@C composite materials were successfully fabricated by a hydrothermal method using the V2O5, H2C2O4·2H2O and coal tar pitches dissolved in water. It is observed that changing the dosages of coal tar pitches dissolved in water can lead to the various carbon contents in V2O3@C composite materials, resulting in the V2O3@C composite materials possessing different electrochemical performances. As a result, when the carbon content is adjusted to 42.7 %, the prepared V2O3@C-2.0 shows more excellent electrochemical performances than other prepared V2O3@C composite materials. For instance, the V2O3@C-2.0 exhibits the high Li+ storage capacity at 580.2 mAh g−1, after charge-discharge was carried out 100 cycles at 0.1 A g−1. Surprisingly, the V2O3@C-2.0 still shows the Li+ storage capacity at 234 mAh g−1, after 500 cycles at 5.0 A g−1. The prepared V2O3@C composite materials show an excellent industrial application perspective because coal tar pitches are the generally industrial crude materials.
抄録全体を表示PDF形式でダウンロード (5157K) HTML形式で全画面表示 -
Akihiro YAMANO, Tatsuya KUBO, Fumiya CHUJO, Naoto YAMASHITA, Takashi M ...2022 年 90 巻 7 号 p. 077003
発行日: 2022/07/06
公開日: 2022/07/06
[早期公開] 公開日: 2022/05/31ジャーナル オープンアクセス HTMLA rubber-derived sulfur composite cathode material for the Li-S battery/Li-ion battery was synthesized by the vulcanization process of butadiene rubber as a polymer source and a large amount of sulfur. In this study, the higher vulcanization temperature of 450 °C and a larger amount of sulfur (rubber : sulfur = 1 : 10, mass/mass) than the mass production process of the rubber were applied. The rubber-derived sulfur composite has a large amount of sulfur, ca. 58 wt% and a stable cycling ability with a capacity of 400 mAh g−1 at 30 °C. The cells consisting of the rubber-derived sulfur composite as the cathode and SiO as the anode displayed a great potential as for the next-generation LIBs because of its both electrodes containing no transition metals, outstanding electrochemical properties and safety performance. The cell showed a superior high and low temperature performance from −20–80 °C for over 100 cycles and a good rate performance in which the capacity of ca. 600 mAh g−1 was obtained at the 5 C-rate at 60 °C. In addition, there were no thermal runaway and no evolution of hydrogen sulfide in the nail penetration test.
抄録全体を表示PDF形式でダウンロード (2028K) HTML形式で全画面表示 -
Mark Adam FERRY, Jun MARUYAMA, Taka-Aki ASOH, Hiroshi UYAMA2022 年 90 巻 7 号 p. 077004
発行日: 2022/07/06
公開日: 2022/07/06
[早期公開] 公開日: 2022/06/09ジャーナル オープンアクセス HTML
J-STAGE DataA green, rapid, and facile synthesis method of activated carbon was developed and optimized using 50 nm MgO nanoparticle (NP)-based pore templating in tandem with an integrated drying and carbonization-activation heat treatment. TEMPO-oxidized cellulose nanofibers (TOCN) were mixed with MgO NPs and KOH, freeze-dried, heat treated first at 350 °C then at 800 °C to get templated activated carbon. Selective pore formation by MgO and KOH resulting in the development of mesopores and micropores, respectively was confirmed. NP-based templating is confirmed to be viable even with continuous heat treatment, and does not affect any transformations during each heat treatment phase. Through optimization for the highest surface area and optimal hierarchical porosity, templated activated carbon had a high specific capacitance of 269 F g−1 at 0.5 A g−1 with 95.92 % cycle stability after 2000 charge-discharge cycles at 10 A g−1.
 抄録全体を表示PDF形式でダウンロード (5677K) HTML形式で全画面表示
抄録全体を表示PDF形式でダウンロード (5677K) HTML形式で全画面表示 -
Ying LUO, Yingshi HUANG, Yanqing LI, Jun FENG2022 年 90 巻 7 号 p. 077005
発行日: 2022/07/09
公開日: 2022/07/09
[早期公開] 公開日: 2022/06/14ジャーナル オープンアクセス HTML
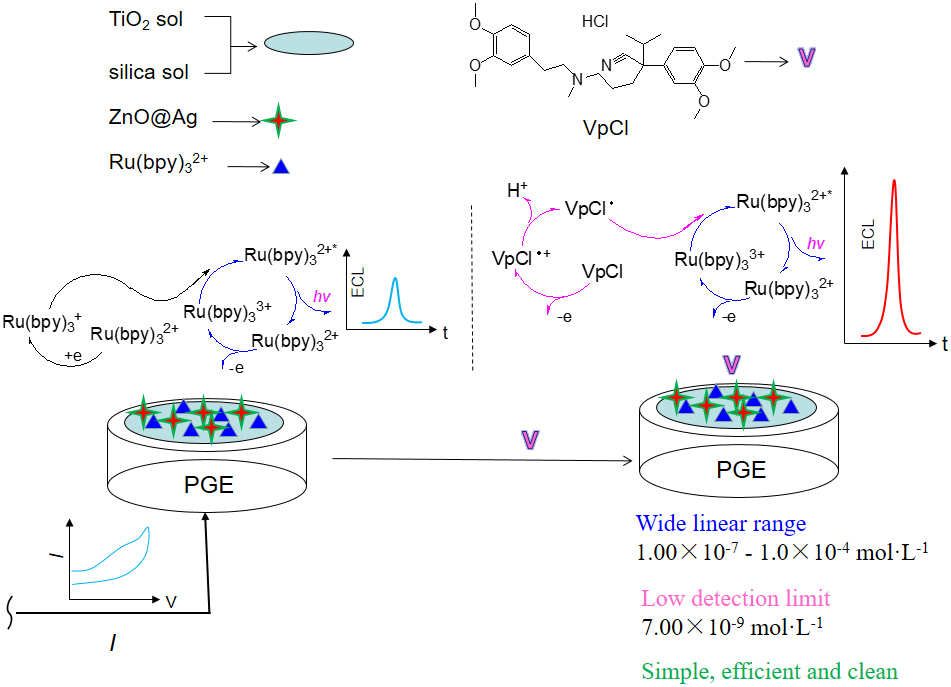
J-STAGE DataA sensitive solid-state electrochemiluminesence (ECL) sensor for verapamil hydrochloride (VpCl) was fabricated based on the film formability of TiO2 sol and electrocatalysis of ZnO@Ag. The immobilization of tris(2, 2′-bipyridine) ruthenium (II) (Ru(bpy)32+) by using TiO2 sol/ZnO@Ag/silica sol composite exhibited stable ECL behaviors, and a sensitive and selective method was developed for VpCl determination. Under the optimal conditions, the ECL response of the sensor showed a linear correlation to the concentration of VpCl in the range of 1.0 × 10−7–1.0 × 10−4 mol L−1 (R2 = 0.9832) with a detection limit of 7.00 × 10−9 mol L−1 (S/N = 3). The RSD of ECL response was 2.07 % for 1.0 × 10−5 mol L−1 VpCl under 10 continuous cyclic scans. The spiked recovery was 97.90–104.50 % for determination of real VpCl sample. The proposed method exhibited selectivity and sensitivity for VpCl, and improved efficiency of Ru(bpy)32+.
 抄録全体を表示PDF形式でダウンロード (2538K) HTML形式で全画面表示
抄録全体を表示PDF形式でダウンロード (2538K) HTML形式で全画面表示 -
Igseon GU, Takuya ISHIDA, Tetsu TATSUMA2022 年 90 巻 7 号 p. 077006
発行日: 2022/07/09
公開日: 2022/07/09
[早期公開] 公開日: 2022/05/17ジャーナル オープンアクセス HTMLChiral plasmonic nanostructures attract much attention because of their potential applications to advanced optical materials and enantioselective sensors. However, the latter has been fabricated on the basis of top-down methods, which take time and cost. Here we developed a one-step method for preparation of chiral gold nanostructures immobilized on an electrode by a simple electrodeposition in the presence of L- or D-cysteine. Opposite circular dichroism (CD) spectra were obtained by using L- or D-cysteine, while achiral structures were deposited for racemic cysteine or in the absence of cysteine. The chirality was attributed to geometries of the nanostructures. Chiral gold nanostructures electrodeposited in the presence of L-cysteine gave higher CD signals to (S)-enantiomer than those to (R)-enantiomer of 1,2-propanediol, and vice versa. TiO2-coated electrodes were also used as a substrate for the electrodeposition of chiral nanostructures, so that the chiral plasmonic electrodes would be employed for optoelectronic and photoelectrochemical applications.
 抄録全体を表示PDF形式でダウンロード (3497K) HTML形式で全画面表示
抄録全体を表示PDF形式でダウンロード (3497K) HTML形式で全画面表示 -
Hironobu HORI, Chikako ISHIKAWA, Atsushi INOISHI, Hikari SAKAEBE, Shig ...2022 年 90 巻 7 号 p. 077007
発行日: 2022/07/30
公開日: 2022/07/30
[早期公開] 公開日: 2022/06/14ジャーナル オープンアクセス HTMLIn a conversion-type electrode material of lithium-ion batteries, a phase separation phenomenon is induced by charge-discharge reaction. In this study, the spatial periodicity of phase-separated nanostructures induced in the discharged ferrous fluoride (FeF2) electrodes were investigated using the small-angle X-ray scattering (SAXS) method. The SAXS results of discharged FeF2 resembled the scattering results of the bicontinuous structures via spinodal decomposition. Thus, the SAXS results showed that the discharged FeF2 electrodes were modulated nanostructures with a spatial periodicity. SAXS measurements also showed that the size of the discharged FeF2 nanostructures was dependent on the cycle number. These SAXS finding on the morphological evolution of the nanoscale structure of conversion electrodes should be useful to reveal the mechanism of conversion reactions.
 抄録全体を表示PDF形式でダウンロード (3521K) HTML形式で全画面表示
抄録全体を表示PDF形式でダウンロード (3521K) HTML形式で全画面表示
- |<
- <
- 1
- >
- >|