
- |<
- <
- 1
- >
- >|
-
Xing Gui WANG, Jie Wen LIU, Hai Liang WANG, Ying Jie DING, Yong Ji GUO2023 年91 巻3 号 p. 037001
発行日: 2023/03/14
公開日: 2023/03/14
[早期公開] 公開日: 2023/02/14ジャーナル オープンアクセス HTMLThe accuracy and connection mode of the equivalent model of retired power battery cells will affect the size of the combined capacity. To this end, this article analyzes series-parallel combination capacity of retired power batteries. First, because an accurate single equivalent circuit model of retired power batteries is necessary for the capacity analysis of the combination, the forgetting factor recursive least squares (FFRLS) algorithm is used to identify the parameters of the model, and the second-order RC equivalent circuit is selected as the single equivalent circuit model of retired power battery. Then the equivalent circuit model is used as the basis, respectively constructing first-parallel-before-series and first-series-before-parallel power battery combination, and fully and unequally arranged the retired power batteries under the two assemblies. Finally, the capacity and distribution characteristics of the two combinations under different arrangements are verified by simulation. The results show that no matter how the arrangement is changed, the maximum capacity of the first-parallel-before-series retired power battery combination is always better than the first-series-before-parallel combination, and the distribution position of the single retired battery in both combinations under the maximum combination capacity is obtained.
抄録全体を表示PDF形式でダウンロード (1548K) HTML形式で全画面表示 -
Mariya YAMAGISHI, Chengchao ZHONG, Daisuke SHIBATA, Mayu MORIMOTO, Yuk ...2023 年91 巻3 号 p. 037002
発行日: 2023/03/16
公開日: 2023/03/16
[早期公開] 公開日: 2023/02/14ジャーナル オープンアクセス HTML
J-STAGE DataAll-solid-state batteries experience irreversible capacity loss particularly in the initial potential cycle, owing to electrolyte decomposition at the electrode/electrolyte interface. A strategy for expanding the oxidation stability of electrolytes is replacing the anion with fluorine. However, fluorine substitution has a negative influence on ionic conductivity. In this study, we introduced trace amounts of fluorine into Li3YCl6 solid electrolytes which exhibit high ionic conductivities and wide potential windows. The effect of replacement on ionic conductivity, oxidation stability, and charge–discharge characteristics were studied. The trace amounts of fluorine in Li3YCl6 did not reduce the conductivity, but improved the apparent oxidation stability. The decomposed product of LiF from the fluorine-substituted electrolyte disturbed the formation of a high-resistance layer at the electrode/electrolyte interface. The initial charge–discharge efficiency of the uncoated LiCoO2 cathode was improved by the trace amount of fluorine replacement in the Li3YCl6 solid electrolyte.
抄録全体を表示PDF形式でダウンロード (2656K) HTML形式で全画面表示 -
Md Saiful ALAM, Isao KAGOMIYA, Ken-ichi KAKIMOTO2023 年91 巻3 号 p. 037003
発行日: 2023/03/22
公開日: 2023/03/22
[早期公開] 公開日: 2023/02/21ジャーナル オープンアクセス HTMLPerovskite oxides obtained from Ba1−xLaxFeO3−δ (BLF) are considered beneficial materials for electrodes of solid oxide fuel cells and oxygen permeation membranes because of their high oxygen permeability, which is a criterion of oxide ion (O2−)-electronic mixed conductivity. In this paper, the prime focus was to understand the oxygen permeation mechanism through surface exchange and bulk diffusion of the Ba0.5La0.5FeO3−δ (BLF55) sample. The permeated oxygen flux displayed higher than that of the typical mixed conductor La0.6Sr0.4Co0.2Fe0.8O3−δ (LSCF), which was explored simultaneously with corresponding oxygen chemical potentials employing an especial experimental setup. This study found that the surface exchange reaction on the oxygen-lean side was the rate-determining step (RDS) of the oxygen permeation below 800 °C, resulting from lower hole concentration on the oxygen-lean side surface. Enhancing the charge transfer from the surface oxygen by increasing hole concentration is a prime important strategy to improve the surface exchange reaction.
 抄録全体を表示PDF形式でダウンロード (4812K) HTML形式で全画面表示
抄録全体を表示PDF形式でダウンロード (4812K) HTML形式で全画面表示 -
Yanjia ZHANG, Benoît D. L. CAMPÉON, Naoaki YABUUCHI2023 年91 巻3 号 p. 037004
発行日: 2023/03/22
公開日: 2023/03/22
[早期公開] 公開日: 2023/02/28ジャーナル オープンアクセス HTML
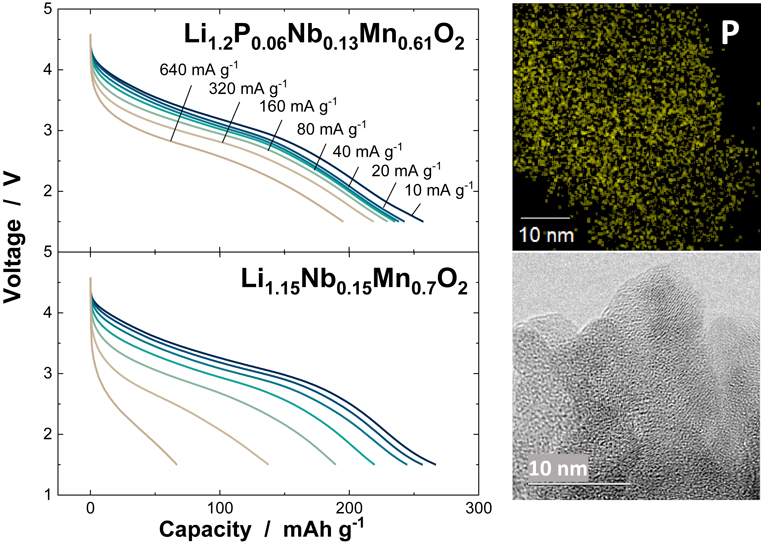
J-STAGE DataA lithium-excess cation-disordered rocksalt oxide, Li1.15Nb0.15Mn0.7O2, is synthesized and tested as positive electrode materials for battery applications. Although nanosized Li1.15Nb0.15Mn0.7O2 delivers a large reversible capacity using cationic/anionic redox reaction, the inferior capacity retention hinders its use for practical applications. Such degradation of electrode reversibility, including electrochemical and structural reversibility, is anticipated to originate from the gradual oxygen loss for the electrode materials with anionic redox. Herein, Li3PO4 is integrated into Li1.15Nb0.15Mn0.7O2 by high-energy mechanical milling, and 7 mol% Li3PO4 integrated Li1.15Nb0.15Mn0.7O2, Li1.2P0.06Nb0.13Mn0.61O2, shows much improved cyclability when compared with the sample without Li3PO4. Approximately 80 % of reversible capacity is retained after 100-cycle test at a rate of 200 mA g−1. Moreover, electrode kinetics are significantly improved by Li3PO4 integration, and Li1.2P0.06Nb0.13Mn0.61O2 delivers a discharge capacity of 200 mA h g−1 at a rate of 640 mA g−1. Li1.2P0.06Nb0.13Mn0.61O2 also shows improved thermal stability at elevated temperatures. From these results, the effectiveness of Li3PO4 integration into nanosized disordered rocksalt oxides with anionic redox is discussed, and this finding leads to the development of metastable high-capacity positive electrode materials for advanced Li-ion batteries.
 抄録全体を表示PDF形式でダウンロード (2701K) HTML形式で全画面表示
抄録全体を表示PDF形式でダウンロード (2701K) HTML形式で全画面表示 -
Yusuke MORINO, Hikaru SANO, Akihiro SHIOTA, Koji KAWAMOTO, Tsukasa TAK ...2023 年91 巻3 号 p. 037005
発行日: 2023/03/24
公開日: 2023/03/24
[早期公開] 公開日: 2023/03/01ジャーナル オープンアクセス HTMLAll-solid-state batteries (ASSBs) using sulfide solid electrolytes (SEs) are attractive candidates as next-generation energy devices having longer lifetimes than liquid-type lithium-ion batteries (LIBs) using organic solvents. Sulfide SEs are known that to suffer a decrease in their ionic conductivity and generate toxic hydrogen sulfide when exposed to moisture even in an environment such as in a dry room. However, the influence of the exposure to moisture on the ASSB cell performance has not been fully elucidated so far. Aiming at filling this gap of knowledge, this paper describes the investigation of the influence of moisture on the durability of an ASSB positive electrode with sulfide SE unexposed or exposed to dry-room-simulated air with dew point of −20 °C in this study. After the cell durability evaluation, time-of-flight secondary-ion mass spectrometry (ToF-SIMS) measurements were performed on positive electrode, and a characteristic degradation mode was observed in the cell using the exposed SE.
 抄録全体を表示PDF形式でダウンロード (1794K) HTML形式で全画面表示
抄録全体を表示PDF形式でダウンロード (1794K) HTML形式で全画面表示 -
Yuko YOKOYAMA, Mitsuo KAWASAKI, Takeshi ABE, Zempachi OGUMI, Kenji KAN ...2023 年91 巻3 号 p. 037006
発行日: 2023/03/24
公開日: 2023/03/24
[早期公開] 公開日: 2023/03/01ジャーナル オープンアクセス HTML
J-STAGE DataFluoride shuttle batteries are expected to be innovative with high energy density superior to lithium-ion ones as on-board power-source for electric vehicles. However, the low solubility of fluoride ion in organic solvents makes it difficult to choose electrolytes. Kawasaki et al. have reported that no precipitation appears in γ-butyrolactone solution of CsF at high concentrations in the presence of excess amounts of Li+ or Mg2+ cation [M. Kawasaki, et al., J. Electrochem. Soc., 169, 110508 (2022).]. Such solutions are called hybrid electrolyte ones. In this study, we performed conductometric titration of Li+- or Mg2+-containing solutions with CsF solutions to elucidate the ion equilibrium. The titration curves were analyzed on models including precipitation of LiF or MgF2 and formation of Li2F+ and LiF2− triple ion or MgF+ associated one to evaluate the corresponding equilibrium constants. The results indicate that no precipitation appears in certain ranges of the F−/Li+ or F−/Mg2+ molar ratio by the triple/associated ion formation. Details of the conductometric analysis are discussed together with some problems involved in the proposed method.
 抄録全体を表示PDF形式でダウンロード (1467K) HTML形式で全画面表示
抄録全体を表示PDF形式でダウンロード (1467K) HTML形式で全画面表示 -
Akihisa TSUCHIMOTO, Masashi OKUBO, Atsuo YAMADA2023 年91 巻3 号 p. 037007
発行日: 2023/03/25
公開日: 2023/03/25
[早期公開] 公開日: 2023/02/28ジャーナル オープンアクセス HTML
J-STAGE DataThe practical application of Li-rich cathode materials exhibiting higher energy density with oxygen redox activity requires improved cycle performance and energy efficiency. Since several conditions such as the amount of excess lithium, transition metal species, and cutoff voltage influence the electrochemical properties of Li-rich cathode materials, comprehensive determination of the optimal conditions often rely on repeating empirical try error processes. Here, the dominant factors in the energy density of Li-rich cathode materials were analyzed by constructing machine learning prediction models based on well-controlled experimental data for simplicity. Choosing a moderate amount of excess lithium and increasing the cobalt contents are the keys to achieve high energy density in long-term cycles.
 抄録全体を表示PDF形式でダウンロード (5523K) HTML形式で全画面表示
抄録全体を表示PDF形式でダウンロード (5523K) HTML形式で全画面表示 -
Ryoichi TATARA, Yosuke UGATA, Shuhei MIYAZAKI, Natsuki KISHIDA, Shohei ...2023 年91 巻3 号 p. 037008
発行日: 2023/03/25
公開日: 2023/03/25
[早期公開] 公開日: 2023/03/01ジャーナル オープンアクセス HTML
J-STAGE DataHighly concentrated Li salt/aprotic solvent solutions are promising electrolytes for next-generation batteries. Understanding the Li+ ion transport process is crucial for designing novel battery electrolytes. In this study, we systematically investigated the phase behavior, solvate structures, and Li+ transport properties of binary mixtures comprising lithium bis(trifluoromethanesulfonyl)amide (LiTFSA) and various sulfones, such as sulfolane (SL), 3-methyl sulfolane (MSL), dimethyl sulfone (DMS), ethyl methyl sulfone (EMS), and ethyl isopropyl sulfone (EiPS). Except for the MSL system, the [LiTFSA]/[sulfone] = 1/2 mixtures remained in a liquid state at room temperature, thus enabling a systematic comparison of the Li+ transport properties in the highly concentrated electrolytes. In highly concentrated liquid electrolytes, Li+ ions diffuse by exchanging ligands (sulfone and TFSA). Li+ ions diffuse faster than TFSA in all electrolytes except the EiPS-based electrolyte at a composition of [LiTFSA]/[sulfone] = 1/2, resulting in high Li+ transference numbers. SL-based electrolytes show higher ionic conductivity and Li+ transference numbers than other sulfone-based electrolytes. Consequently, sulfone solvents with compact molecular sizes and low energy barriers of conformational change are favorable for enhancing the Li+ ion transport in the electrolytes.
 抄録全体を表示PDF形式でダウンロード (1967K) HTML形式で全画面表示
抄録全体を表示PDF形式でダウンロード (1967K) HTML形式で全画面表示 -
Wataru YOSHIDA, Akira NASU, Kota MOTOHASHI, Masahiro TATSUMISAGO, Atsu ...2023 年91 巻3 号 p. 037009
発行日: 2023/03/31
公開日: 2023/03/31
[早期公開] 公開日: 2023/03/02ジャーナル オープンアクセス HTMLHard carbon is a promising negative electrode material for sodium-ion batteries that operate at low potentials. However, reversible and high-capacity charging and discharging in all-solid-state sodium batteries with hard carbon electrodes using sulfide solid electrolytes have not been reported. This study reports that reductive decomposition of the sulfide solid electrolyte occurs at both the negative composite electrode and the interface between the negative electrode layer and the solid electrolyte layer. In the first cycle, the all-solid-state cell with a composite electrode containing a Na3PS4 solid electrolyte exhibited a large irreversible capacity of 561 mAh g−1 because of the reductive decomposition of Na3PS4 to Na2S and Na3P. The use of a Na3BS3 glass electrolyte with reduction stability can lead to the successful charging and discharging of all-solid-state cell that utilizes hard carbon. This glass electrolyte can serve as a solid electrolyte for the negative composite electrode and as a buffer layer between the negative electrode layer and the solid electrolyte layer. Hence, it can also help suppress the irreversible capacity of the cell to 122 mAh g−1.
抄録全体を表示PDF形式でダウンロード (2982K) HTML形式で全画面表示 -
Ryohei KURIHARA, Nana SHINADA, Hideyuki MORIMOTO2023 年91 巻3 号 p. 037010
発行日: 2023/03/31
公開日: 2023/03/31
[早期公開] 公開日: 2023/03/04ジャーナル オープンアクセス HTMLAmorphous materials (a-LAGP(x = 0.5)) with the composition of Li1.5Al0.5Ge1.5(PO4)3 were prepared by mechanochemical synthesis method. Crystalline compounds with LiGe2(PO4)3-based NASICON-type structure were formed by heat treatment of a-LAGP(x = 0.5) at 400–700 °C in air. Solid electrolytes prepared by the heat treatment of pelletized-type a-LAGP(x = 0.5) and MgO-modified a-LAGP(x = 0.5) at 700 °C exhibited the lithium ion conductivities on the order of 10−5–10−4 S cm−1 at room temperature. The addition of MgO into a-LAGP(x = 0.5) increased the electrical conductivity. Lithium ion conductors prepared by low-temperature heat treatment of pelletized-type amorphous materials in the Li2O-Al2O3-GeO2-P2O5 system would be promising as solid electrolytes for all-solid-state batteries.
抄録全体を表示PDF形式でダウンロード (2896K) HTML形式で全画面表示
- |<
- <
- 1
- >
- >|