
- Issue 4 Pages 267-
- Issue 3 Pages 185-
- Issue 2 Pages 113-
- Issue 1 Pages 1-
- |<
- <
- 1
- >
- >|
-
Katsuhiro YAMAMOTO2019Volume 76Issue 1 Pages 1-2
Published: January 25, 2019
Released on J-STAGE: January 25, 2019
JOURNAL FREE ACCESSDownload PDF (428K)
-
 Takahiro SATO, Rintaro TAKAHASHI2019Volume 76Issue 1 Pages 3-22
Takahiro SATO, Rintaro TAKAHASHI2019Volume 76Issue 1 Pages 3-22
Published: January 25, 2019
Released on J-STAGE: January 25, 2019
Advance online publication: December 12, 2018JOURNAL FREE ACCESSWe overview both theoretical and experimental studies on the following kinetics of block copolymer micelles formed in a selective solvent reported so far: 1) the exchange kinetics of block copolymer chains among micelles was investigated by time-resolved small-angle neutron scattering for deuterated block copolymer micelles mixed with corresponding non-labeled micelles; 2) the growth of the micelles after a temperature jump was studied by time-resolved light scattering; 3) the nucleation-growth kinetics of the block copolymer micelles in solution was investigated by light and small-angle X-ray scattering using a stopped flow apparatus; 4) the morphology transition kinetics from cylindrical micelles to spherical micelles of block copolymers was studied by transmittance electron microscopy and time-resolved small-angle X-ray scattering.
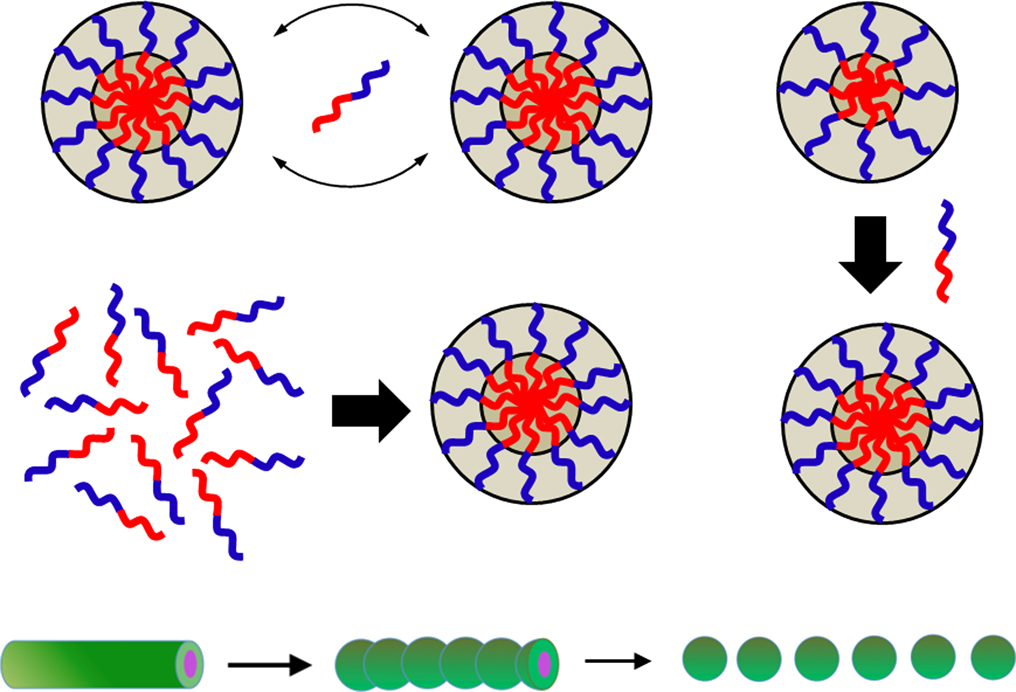 View full abstractDownload PDF (4026K)
View full abstractDownload PDF (4026K)
-
Hao-xuan GUO, Keisuke YOSHIDA, Hiroyuki AOTA2019Volume 76Issue 1 Pages 23-32
Published: January 25, 2019
Released on J-STAGE: January 25, 2019
Advance online publication: December 27, 2018JOURNAL FREE ACCESSA new type of pseudo-living polymerization by continuous addition of the monomers in the addition-condensation of 1-methylpyrrole and aldehydes has been studied. This polymerization is able not only to control the structure of the polymer such as block and branched, but also to introduce functional groups such as energy donors and acceptors. We show two types of polymers that are used in photochemical studies, which suggest that the materials prepared from this new type of polymerization may be useful in artificial photosynthesis. The first is an A, B-block amphiphilic polymer in which the energy donor and acceptor are each in the different blocks. This polymer shows an interphase photoinduced energy transfer in a micellar system. The second polymer is a branched polymer, with a high number of energy donor units in the branches and a low number of energy acceptor units in its center that is also bearing electron donor-acceptor units. The polymer combines efficient light harvesting with photoinduced electron transfer.
 View full abstractDownload PDF (1492K)
View full abstractDownload PDF (1492K) -
Mikihito TAKENAKA, Hirokazu HASEGAWA, Yi-Chin WANG, Myung Im KIM2019Volume 76Issue 1 Pages 33-43
Published: January 25, 2019
Released on J-STAGE: January 25, 2019
Advance online publication: November 14, 2018JOURNAL FREE ACCESSWe focus on the formation of the complex network microdomain morphologies ob block copolymers by different methods. Precise control of composition in polystyrene-block-polyisoprene (SI) was attained by anionic polymerization of styrene and isoprene or by addition of homopolymer into neat SI, and enabled us to form the Fddd structure. An ordered bicontinuous double-diamond (OBDD) morphology was found in Polystyrene-block-(poly-4-vinylphenyldimethylvinylsilane-graft-polyisoprene), a block-graft copolymer. The grafted polyisoprene chains induce the frustration of polystyrene chains and thus the effects of the specific interface are more dominant that those of the packing frustration in the formation of the morphology and the OBDD phase is thus stabilized. Ordered tricontinuous double-diamond morphology (OTDD) was formed in cast films of polystyrene-block-polyisoprene-block-polydimethylsiloxiane a triblock terpolymer from toluene solution, though the OTDD structure is not an equilibrium structure in triblock terpolymers. Two-step microphase separation occurs during solvent cast. Since the kinetic barrier of the transition from cylinder to gyroid is higher than that from cylinder to OTDD, the metastable OTDD was formed.
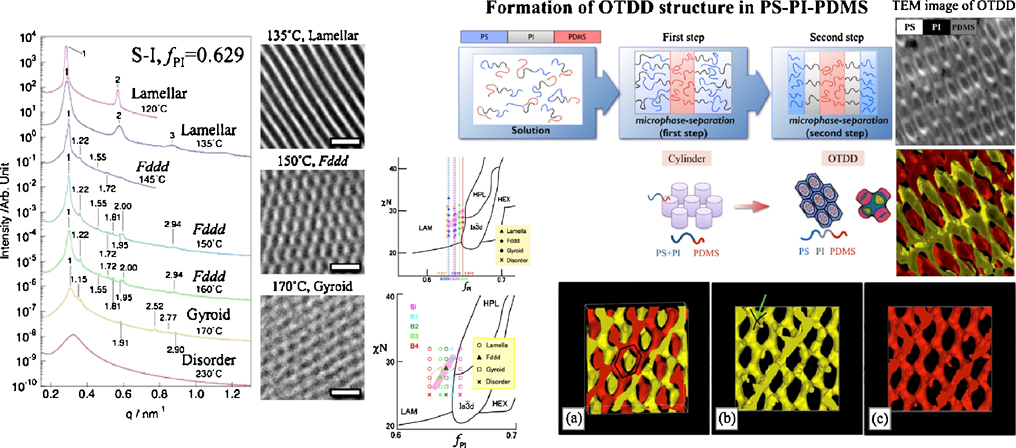 View full abstractDownload PDF (5430K)
View full abstractDownload PDF (5430K)
-
Takashi UNEYAMA2019Volume 76Issue 1 Pages 44-51
Published: January 25, 2019
Released on J-STAGE: January 25, 2019
Advance online publication: October 11, 2018JOURNAL FREE ACCESSWe propose a highly coarse-grained, self-consistent field (SCF) theory for simulations of symmetric diblock copolymers, based on the soft dumbbell model. We apply the standard derivation method of the SCF model to the soft dumbbell model, and derive the set of self-consistent equations. We perform the SCF simulations in one dimension, based on the derived model, and show that the SCF model can reproduce microphase separation structures properly. We theoretically consider the properties of the model and discuss possible applications.
 View full abstractDownload PDF (844K)
View full abstractDownload PDF (844K) -
Keita SHIRAISHI, Kazuhiko ISHIHARA, Shin-ichi YUSA2019Volume 76Issue 1 Pages 52-60
Published: January 25, 2019
Released on J-STAGE: January 25, 2019
Advance online publication: November 01, 2018JOURNAL FREE ACCESSAmphiphilic diblock copolymer (PMPC-PBMA) composed of biocompatible water-soluble poly(2-methacryloyloxyethyl phosphorylcholine) (PMPC) and hydrophobic poly(n-butyl methacrylate) (PBMA) was synthesized via reversible addition-fragmentation chain transfer (RAFT) radical polymerization. Degrees of polymerization for PMPC and PBMA were 95 and 181, respectively. PMPC-PBMA can dissolve in water to form core-shell ellipsoidal polymer micelles. The core of the micelle can incorporate hydrophobic cyanine dye (Cyd) which can absorb light at near infrared (NIR) wavelength. The average hydrodynamic radius (Rh) for Cyd-loaded polymer micelles was about 103 nm in water. Exothermic behavior of the aqueous solution can be observed when NIR light irradiates Cyd-loaded polymer micelles in water. It is expected that the Cyd-loaded polymer micelles can be applied to photo thermal therapy (PTT).
 View full abstractDownload PDF (3058K)
View full abstractDownload PDF (3058K) -
 Yuushou NAKAYAMA, Tomohito UNO, Takeshi SHIONO, Hiroyuki SHIRAHAMA2019Volume 76Issue 1 Pages 61-67
Yuushou NAKAYAMA, Tomohito UNO, Takeshi SHIONO, Hiroyuki SHIRAHAMA2019Volume 76Issue 1 Pages 61-67
Published: January 25, 2019
Released on J-STAGE: January 25, 2019
Advance online publication: December 28, 2018JOURNAL FREE ACCESSSequential copolymerization of l-lactide (LLA) with ε-caprolactone (CL) followed by that of d-lactide (DLA) with CL was carried out to synthesize Poly(LLA-r-CL)-b-Poly(DLA-r-CL). The obtained Poly(LLA-r-CL)-b-Poly(DLA-r-CL) showed only one Tm ca. 30°C higher than that of Poly(LLA-r-CL), indicating selective formation of stereocomplex crystals. The stereoblock Poly(LLA-r-CL)-b-Poly(DLA-r-CL) exhibited high elongation at break similarly to homochiral Poly(LLA-r-CL). Their biodegradation test using proteinase K showed lower degradability than that of homochiral Poly(LLA-r-CL).
 View full abstractDownload PDF (802K)
View full abstractDownload PDF (802K) -
Masaki KINOSHITA, Daisuke KUGIMOTO, Kosuke MORIMOTO, Kazuyuki ITO, Ken ...2019Volume 76Issue 1 Pages 68-73
Published: January 25, 2019
Released on J-STAGE: January 25, 2019
Advance online publication: November 21, 2018JOURNAL FREE ACCESSpH-triggered structural change of polymer micelles composed of poly(methacrylic acid)-block-poly(2-bromethyl methacrylate) (PMAA-b-PBrEMA) was investigated with anomalous small-angle X-ray scattering (ASAXS) near the Br K edge. ASAXS revealed that the size of the hydrophobic core and the aggregation number of PMAA-b-PBrEMA micelles drastically decreased with an increase in the pH from 4.8 to 12.5. It was concluded that the drastic reduction of the size of PMAA-b-PBrEMA micelles in alkaline conditions was caused by the reduced electrostatic repulsion between anionic PAA chains in the corona layer of the micelles.
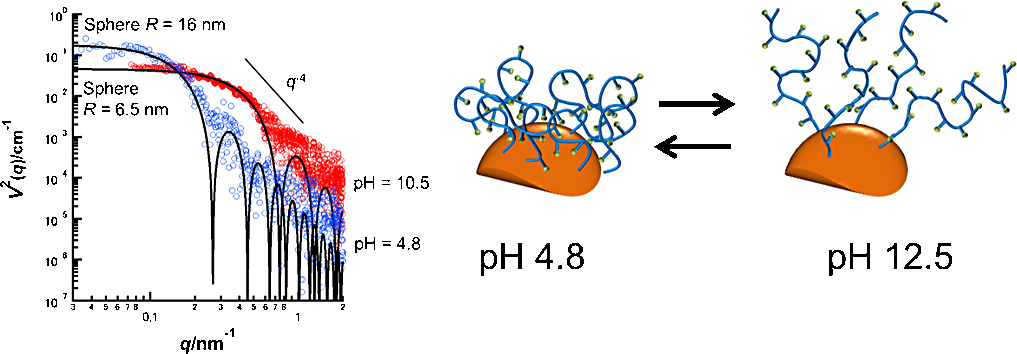 View full abstractDownload PDF (1534K)
View full abstractDownload PDF (1534K)
-
Akihito HASHIDZUME, Yoshiro MITSUKAMI, Shin-ichi YUSA, Yotaro MORISHIM ...2019Volume 76Issue 1 Pages 74-78
Published: January 25, 2019
Released on J-STAGE: January 25, 2019
Advance online publication: October 23, 2018JOURNAL FREE ACCESSAn unsymmetric diblock copolymer b-Q57A97 of (ar‑vinylbenzyl)trimethylammonium chloride (Q) and N,N-dimethylvinylbenzylamine (A), synthesized by reversible addition-fragmentation chain transfer radical polymerization, and its molecular assemblies were characterized by static and dynamic light scattering and steady state fluorescence using N-phenyl-1-naphthylamine (PNA) as a probe. Scanning electron microscopy observations indicated that b-Q57A97 formed a unique fern leaf-like morphology in the presence of NaCl.
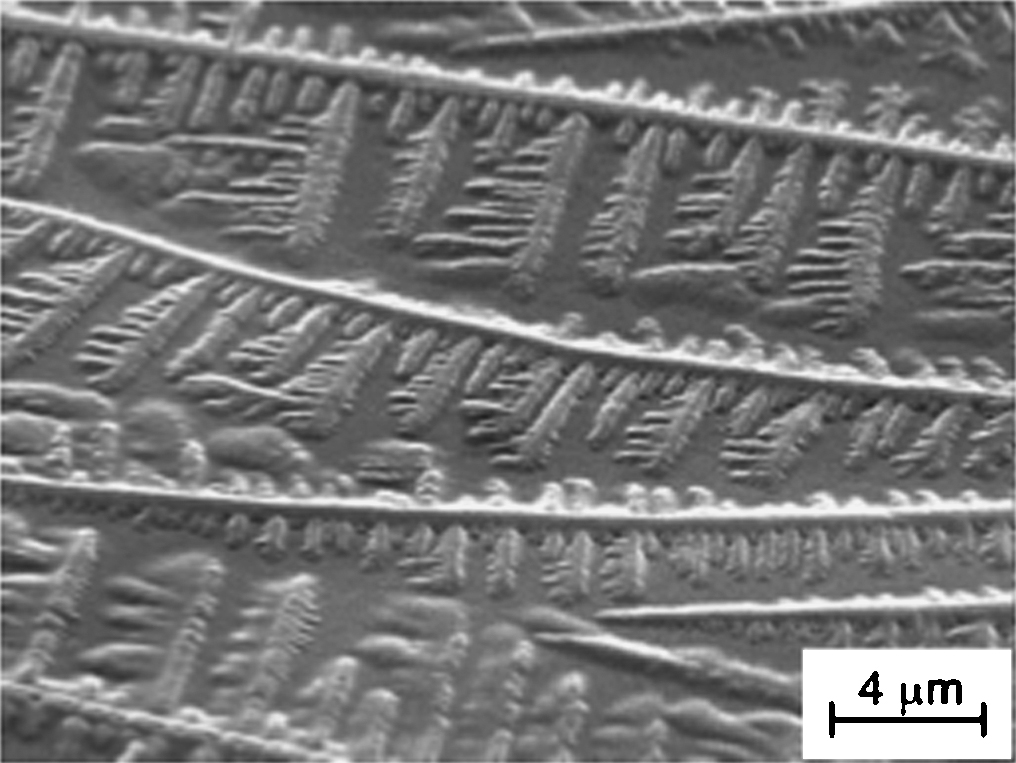 View full abstractDownload PDF (1894K)
View full abstractDownload PDF (1894K) -
Shoichi ISHIMOTO, Sho HIRAI, Hideaki OBUCHI, Shigeru YAO2019Volume 76Issue 1 Pages 79-82
Published: January 25, 2019
Released on J-STAGE: January 25, 2019
Advance online publication: October 30, 2018JOURNAL FREE ACCESSPolyethylene (PE) is known as the typical plastic whose surface properties are very difficult to modify and it is believed that it is impossible to modify the surface properties chemically. However, recently, Yao had clearly shown that side chain crystalline block copolymer (SCCBC) has an ability to modify the PE surface property chemically. In this study, by using the SCCBC, we had tried to stain the very high molecular weight PE fiber that is known to be the most difficult product with respect to modifying the surface property, not only chemically but also physically. From the results, the high molecular weight PE fiber can be dyed by using the SCCBC and it had shown very high durability. This study shows that the application area of SCCBC is very wide and useful.
 View full abstractDownload PDF (2034K)
View full abstractDownload PDF (2034K)
-
Hiroki UEOKA, Osamu SHIMOMURA, Ryoki NOMURA2019Volume 76Issue 1 Pages 83-89
Published: January 25, 2019
Released on J-STAGE: January 25, 2019
Advance online publication: October 29, 2018JOURNAL FREE ACCESSPhysically cross-linked chitosan-poly(N-isopropylacrylamide) (CS-PNIPA) gel with trisodium citrate (CIT), sodium tripolyphosphate (TPP), and sodium alginate (Alg) as cross-linkers were prepared to obtain the cross linked gels of CIT@CS-PNIPA, TPP@CS-PNIPA and Alg@CS-PNIPA. The elastic moduli of these gels were 18.9 N/mm2 for CIT@CS-PNIPA, 86.6 N/mm2 for TPP@CS-PNIPA, and <0.01 N/mm2 for Alg@CS-PNIPA. The release ratio of cyanocobalamin (VB12) encapsulated into CIT@CS-PNIPA and TPP@CS-PNIPA was evaluated at 25°C and 40°C. At 40°C, higher than the LCST of PNIPA, for 30 min, the VB12 encapsulated was quantitatively released from both, CIT@CS-PNIPA and TPP@CS-PNIPA. At 25°C, lower than the LCST of PNIPA, for 30 min, the releae of VB12 was 70% for CIT@CS-PNIPA and 10% for TPP@CS-PNIPA. The gel of TPP@CS-PNIPA was better as a thermo-responsive gel of drug delivery carrier.
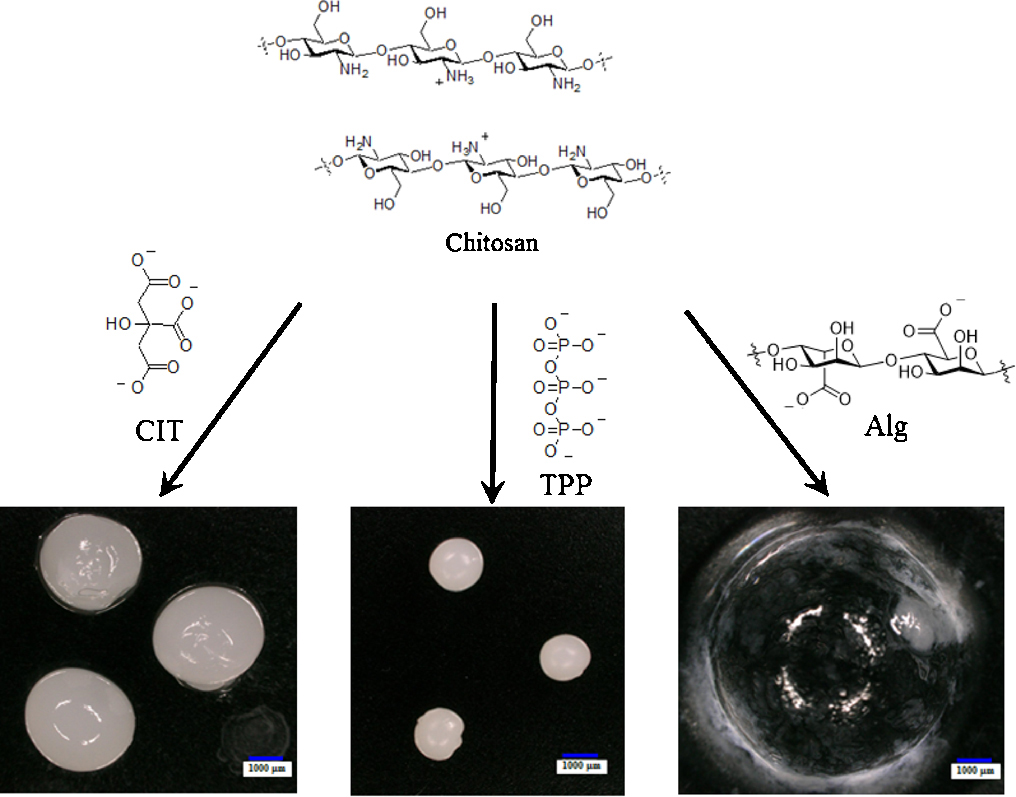 View full abstractDownload PDF (1895K)
View full abstractDownload PDF (1895K) -
Atsuyuki NOWAKI, Hiroki OTA, Takafumi OUCHI, Kentaro MATSUMOTO, Chi Ho ...2019Volume 76Issue 1 Pages 90-97
Published: January 25, 2019
Released on J-STAGE: January 25, 2019
Advance online publication: December 05, 2018JOURNAL FREE ACCESSIn order to develop antistatic composites consisting of bamboo micro-whiskers (BµF) and polypropylene (PP), surface properties of superheated steam (SHS)-treated materials were analyzed to find good hydrophobicity by mainly lignin coating. The SHS-treated BµF contributed to decrease in surface-resistivity of composites compared to volume-resistivity. Moreover, the composites including expandable graphite (EG) as a flame-retardant showed excellent antistatic properties. Although addition of a compatibilizer [maleic anhydride-modified PP (MAPP)] suppressed the antistatic effect of BµF, synergistic antistatic effect of BµF/EG overcame the suppression effect, resulting in a composite BµF/PP/EG/MAPP having good antistatic properties as well as flame-resistance and good mechanical properties.
 View full abstractDownload PDF (3089K)
View full abstractDownload PDF (3089K) -
Michio URUSHISAKI, Tamotsu HASHIMOTO, Toshikazu SAKAGUCHI2019Volume 76Issue 1 Pages 98-107
Published: January 25, 2019
Released on J-STAGE: January 25, 2019
Advance online publication: December 14, 2018JOURNAL FREE ACCESSTo develop an epoxy resin having a high glass transition temperature and heat resistance, copolymerization of an epoxy group-containing vinyl ether and a maleimide derivative was carried out. Radical copolymerization of 2-vinyloxyethylglycidyl ether (VEGE) and N-phenylmaleimide (NPMI) with AIBN were carried out in benzene or THF to give polymers having a molecular weight of 120,000 to 250,000 (in case of benzene) and a molecular weight of about 6,000 (in case of THF) in high yield, respectively. On the other hand, the copolymerization in the absence of AIBN hardly progressed in benzene, but in THF the polymers with a molecular weight of about 5,000 were obtained in high yield. 1H NMR spectroscopy showed that the structure of the products is a copolymer composed of VEGE units and NPMI units. MALDI-TOF-MS spectroscopy revealed that the VEGE units and NPMI units are arranged mainly in an alternative manner. The glass transition temperature (Tg) of the copolymers greatly varied depending on the copolymer composition and remarkably increased as the content of NPMI units increased. The thermal decomposition temperature (Td) of the copolymers was about 300°C or higher depending on the content NPMI units. By curing the reactive epoxy groups in the copolymers with multifunctional aromatic amines, novel heat-resistant epoxy cured resins with high Tg were obtained.
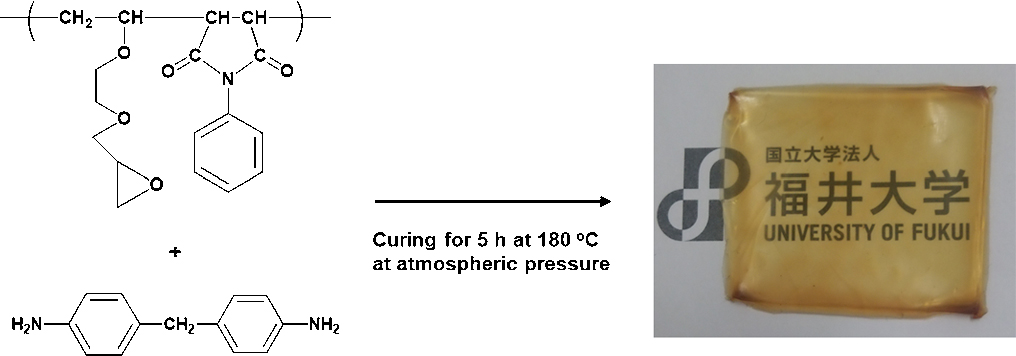 View full abstractDownload PDF (3110K)
View full abstractDownload PDF (3110K)
-
Kazuyuki HIRAOKA, Yu IWAKABE, Kazumi YAMADA, Masato OSHIMA2019Volume 76Issue 1 Pages 108-111
Published: January 25, 2019
Released on J-STAGE: January 25, 2019
Advance online publication: November 02, 2018JOURNAL FREE ACCESSPolymer networks composed of liquid-crystalline polyesters were synthesized with two kinds of titanium alkoxide catalysts, titanium tetrabutoxide and titanium tetraisopropoxide. We confirmed that titanium tetrabutoxide worked at a lower temperature in the reaction for the formation of a polymer network; a compound containing a gel component of 84 weight percent was obtained in the synthesis at 210°C with titanium tetrabutoxide catalyst, while that containing a gel component of 44 weight percent was obtained with titanium tetraisopropoxide at 230°C.
 View full abstractDownload PDF (962K)
View full abstractDownload PDF (962K)
- |<
- <
- 1
- >
- >|