
- |<
- <
- 1
- >
- >|
-
2021 Volume 61 Issue 9 Pages Cover-
Published: September 15, 2021
Released on J-STAGE: September 15, 2021
JOURNAL OPEN ACCESSDownload PDF (456K) -
2021 Volume 61 Issue 9 Pages Editorial-
Published: September 15, 2021
Released on J-STAGE: September 15, 2021
JOURNAL OPEN ACCESSDownload PDF (543K) -
2021 Volume 61 Issue 9 Pages Contents-
Published: September 15, 2021
Released on J-STAGE: September 15, 2021
JOURNAL OPEN ACCESSDownload PDF (297K)
-
Hideki OnoArticle type: Preface
2021 Volume 61 Issue 9 Pages 2323
Published: September 15, 2021
Released on J-STAGE: September 15, 2021
JOURNAL OPEN ACCESS FULL-TEXT HTMLDownload PDF (212K) Full view HTML -
Yoshihiko Higuchi, Hideki Ono, Katsuyoshi OkumotoArticle type: Review
2021 Volume 61 Issue 9 Pages 2324-2330
Published: September 15, 2021
Released on J-STAGE: September 15, 2021
Advance online publication: June 27, 2021JOURNAL OPEN ACCESS FULL-TEXT HTMLControlling inclusion content in high chromium steel is very important to prevent submerged entry nozzle from clogging in continuous casting and avoid the negative impacts of inclusions on steel properties. Therefore, effects of temperature and content of elements on phase stability diagram should be clarified in chromium bearing steel. However, the effect of chromium content on boundaries of MgO, MgO∙Al2O3 and Al2O3 in phase stability diagram are much different among the researchers. The direction of boundaries shift is affected by chromium content differently. Temperature dependencies of deoxidation equilibrium constants below 1873 K are also scattered. Calcium, which is used to avoid the negative effect of MgO∙Al2O3 inclusion, enlarges liquid region in phase stability diagram. However, the region replaced by liquid oxide is understood differently in low alloyed steel and high chromium steel. In TiOX–Al2O3–MgO system inclusion, commercial thermochemical software predicts that boundaries of Ti2O3, Ti3O5, Al2O3 and TiOx–Al2O3 shift toward lower titanium content in high chromium steel. However, the calculated phase stability diagrams vary among studies even in liquid iron or low alloyed steel. Therefore, equilibrium experiments under various conditions and reliable technique of thermodynamic calculation with high accuracy are desired.
View full abstractDownload PDF (1282K) Full view HTML -
Hiroshi Fukaya, Seika Nakajima, Jonah Gamutan, Shigeru Suzuki, Koji Ka ...Article type: Regular Article
2021 Volume 61 Issue 9 Pages 2331-2339
Published: September 15, 2021
Released on J-STAGE: September 15, 2021
JOURNAL OPEN ACCESS FULL-TEXT HTMLThe aluminum deoxidation equilibrium in molten Fe-10 to 40mass%Cr-8mass%Ni and Fe-18mass%Cr-8 to 30mass%Ni alloys was experimentally determined at 1873 K and 1773 K to obtain the thermodynamic parameters at both temperatures, corresponding to the refining and casting processes, respectively. Thermodynamic analysis on Al deoxidation was carried out based on the sub-regular solution model using a Redlich–Kister type polynomial. Fe–Al, Ni–Al, Cr–Al and Fe–Cr–Ni interaction parameters were obtained from experimental results and a thermodynamic assessment. Using these parameters, the Al deoxidation equilibrium over the complete composition range of the Fe–Ni alloy and in more than 50mass%Fe of the Fe–Cr and Fe–Cr–Ni alloys can be calculated for the temperature ranges of both of the refining and casting processes.
 Calculated logK’ of the Al deoxidation equilibrium of the Fe–Cr–Ni system at liquidus temperatures. Fullsize ImageView full abstractDownload PDF (1363K) Full view HTML
Calculated logK’ of the Al deoxidation equilibrium of the Fe–Cr–Ni system at liquidus temperatures. Fullsize ImageView full abstractDownload PDF (1363K) Full view HTML -
Zhi Li, Chisei Kato, Yoshinao KobayashiArticle type: Regular Article
2021 Volume 61 Issue 9 Pages 2340-2344
Published: September 15, 2021
Released on J-STAGE: September 15, 2021
JOURNAL OPEN ACCESS FULL-TEXT HTMLFor a better thermodynamic understanding of the optimization of molten slag in the stainless-steel refining process, the solubility of chromium oxide in the CaO–SiO2–Cr2O3 slag system was investigated. A chemical equilibrium technique was employed in which molten slag and solid Cr2O3 pellets were equilibrated under a regulated oxygen partial pressure (PO2) lower than 10−11 atm at 1873 K. The mass ratio of CaO to SiO2 (XCaO / XSiO2), as an index of slag basicity, was varied from 0.5 to 1.5. A CO–CO2–Ar gas mixture was used, and the oxygen partial pressure was precisely controlled by evaluating possible contamination by oxygen that was inevitably introduced into the gas mixture system. A zirconia oxygen sensor was used to directly measure the oxygen partial pressure for this evaluation. The solubility of chromium oxide increased with decreasing slag basicity and oxygen partial pressure. Accordingly, slag design is a huge prospect toward achieving desirable refining conditions.
View full abstractDownload PDF (554K) Full view HTML -
Yan Lu, Takahiro MikiArticle type: Regular Article
2021 Volume 61 Issue 9 Pages 2345-2354
Published: September 15, 2021
Released on J-STAGE: September 15, 2021
JOURNAL OPEN ACCESS FULL-TEXT HTMLPhase equilibria in Fe–Mn–S and Fe–Cr–S ternary systems at 1843 K were investigated experimentally, respectively. The main characteristic of these two systems at 1843 K was confirmed to be a wide miscibility gap between two liquid phases: molten metal alloy phase and molten sulfide phase. Through metal/sulfide equilibrium method, activity of constituents in sulfide phase were determined in Fe–Mn–S and Fe–Cr–S systems separately. The activity curves of constituents in sulfide phase were estimated by utilizing regular solution model.
View full abstractDownload PDF (1040K) Full view HTML -
Yan Lu, Takahiro MikiArticle type: Regular Article
2021 Volume 61 Issue 9 Pages 2355-2359
Published: September 15, 2021
Released on J-STAGE: September 15, 2021
JOURNAL OPEN ACCESS FULL-TEXT HTMLPhase equilibria in Fe–Cr–Mn–S quaternary system at 1843 K were investigated experimentally. Two liquid phases: molten metal alloy phase and molten sulfide phase were in equilibrium in this system at 1843 K. The equilibrium relations between molten metal alloy and sulfide phases were experimentally measured. By using metal/sulfide equilibrium method, activity of constituents in molten MnS–CrS–FeS sulfide phase were determined. By utilizing regular solution model, activity curves of constituents in sulfide phase were estimated.
View full abstractDownload PDF (744K) Full view HTML -
Yan Lu, Takahiro MikiArticle type: Regular Article
2021 Volume 61 Issue 9 Pages 2360-2369
Published: September 15, 2021
Released on J-STAGE: September 15, 2021
JOURNAL OPEN ACCESS FULL-TEXT HTMLThermodynamic property of solid MnS–CrS–FeS system was determined based on the combination between thermodynamic properties of liquid MnS–FeS, CrS–FeS, and MnS–CrS–FeS phase determined by the authors and reported phase diagram of MnS–FeS, CrS–FeS and MnS–CrS system. The determined parameters were verified by comparison with experimental results of equilibrium relationship between metal/sulfide in Fe–Cr–Mn–S system at 1793 K. By utilizing the determined parameters, phase equilibria involving sulfide phase in liquid Fe–Cr–Mn–S system was established. According to the phase equilibria information, controllability of MnS–CrS–FeS sulfide phase in typical stainless steel during solidification was evaluated.
View full abstractDownload PDF (1580K) Full view HTML -
Katsuyoshi Okumoto, Kengo Kato, Hideki Ono, Yoshihiko HiguchiArticle type: Regular Article
2021 Volume 61 Issue 9 Pages 2370-2380
Published: September 15, 2021
Released on J-STAGE: September 15, 2021
JOURNAL OPEN ACCESS FULL-TEXT HTMLDemands for cleanliness of high chromium steel have been increasing. In steel refining process, aluminum is usually added in molten steel as a deoxidizing agent. As a result, such inclusions as alumina (Al2O3) and spinel (MgO∙Al2O3) are formed, which cause fatigue failures and surface defects. Therefore, it is important to understand the conditions of the inclusions which form in high chromium steel, and to reduce their harmful effects on steel qualities. In this work, to begin with, thermodynamic conditions of MgO and MgO∙Al2O3 formation in Fe-17mass%Cr molten steel at 1873 K were investigated. The results showed that MgO is more stable in high chromium steel than in plain steel. The boundary of the stable condition of MgO and MgO∙Al2O3 shifts toward higher Al and lower Mg contents in high Cr steel. This cause is judged to be the effect of thermodynamic interaction between Cr and Mg. The interaction parameter of Cr on Mg was estimated to be 0.040 so that the boundary of stable condition of MgO and MgO∙Al2O3 can be explained. Moreover, phase stability diagram of Fe–Cr–Al–Ca–Mg–O system at 1873 K was developed to estimate the effect of chromium on the stable condition of MgO, MgO∙Al2O3 and CaO–MgO–Al2O3(l). Subsequently, the variations of inclusions which formed in Fe-17mass%Cr molten steel were also investigated at 1873 K. The variations of inclusions in molten Fe–Cr steel were reasonably explained by considering the stable conditions of MgO and MgO∙Al2O3 investigated in this work.
View full abstractDownload PDF (1293K) Full view HTML -
Tomoki Furukawa, Noritaka Saito, Kunihiko NakashimaArticle type: Regular Article
2021 Volume 61 Issue 9 Pages 2381-2390
Published: September 15, 2021
Released on J-STAGE: September 15, 2021
Advance online publication: August 25, 2021JOURNAL OPEN ACCESS FULL-TEXT HTMLThe contact angles between three non-metallic inclusion-type oxide substrates, viz. Al2O3, MgO, and MgO·Al2O3, and molten Fe and molten Fe-based stainless steel (Fe–Cr–Ni alloy) were measured using the sessile drop method in Ar atmosphere at 1873 K. The contact angles between molten Fe and oxide substrates ranged between 111° and 117°, while that between molten Fe–Cr–Ni alloy and substrates ranged between 103° and 105°. The angles between the alloy and each of the substrates were smaller than the corresponding values for Fe, which was attributed to the superior wettability of molten Fe–Cr–Ni alloy on the substrates. The wettability of the molten materials is related to the interfacial tension between the molten metals and each substrate. Thus, the interfacial tension between the molten metals and the non-metallic substrates was quantitatively evaluated using Young’s equation and the measured contact angles; the interfacial tension for molten Fe ranged from 1.862 to 2.781 N·m−1, while that for molten Fe–Cr–Ni alloy ranged from 1.513 to 2.286 N·m−1. Owing to the higher reactivity between molten Fe–Cr–Ni alloy and the substrates, the interfacial tension and energy between them were lower than those between molten Fe and the substrates.
View full abstractDownload PDF (2207K) Full view HTML -
Ying Ren, Chunyang Liu, Xu Gao, Lifeng Zhang, Shigeru Ueda, Shin-ya Ki ...Article type: Regular Article
2021 Volume 61 Issue 9 Pages 2391-2399
Published: September 15, 2021
Released on J-STAGE: September 15, 2021
Advance online publication: March 25, 2021JOURNAL OPEN ACCESS FULL-TEXT HTMLDolomite refractories are widely used in the refining process of clean steel and are considered potential sources of Mg and Ca that form MgO·Al2O3 spinel and CaO-containing inclusions. In this study, dolomite refractories were immersed into Al-killed molten steel with either 0.05% Al or 0.25% Al. The dissolution behavior of Mg and Ca from the dolomite refractory was studied, and the inclusion transformation behavior was observed. The results revealed that MgO in the dolomite refractory was reduced by Al in the molten steel, and the Mg content depended on the Al content. On the contrary, CaO barely dissolved into the molten steel even though the Al content increased. After immersion in both the low Al (0.05% Al) and high Al (0.25% Al) steels, an interfacial layer consisting of solid MgO and liquid phase CaO–Al2O3–MgO was formed on the surface of the rods. The initial Al2O3 inclusions gradually changed into Al2O3 saturated MgO–Al2O3 spinel after 60 min in low-Al steel; but were quickly transformed into MgO-saturated MgO–Al2O3 spinel in high Al steel. No CaO-containing inclusions were detected in the molten steel regardless of the immersion time and Al content.
View full abstractDownload PDF (2568K) Full view HTML -
Zilong Qiu, Annelies Malfliet, Muxing Guo, Bart BlanpainArticle type: Regular Article
2021 Volume 61 Issue 9 Pages 2400-2409
Published: September 15, 2021
Released on J-STAGE: September 15, 2021
Advance online publication: June 18, 2021JOURNAL OPEN ACCESS FULL-TEXT HTMLTo predict the clustering of inclusions at the liquid steel and inert gas interface, it is essential to calculate the pairwise attractive (inertial) force on the inclusions correctly. In most of the studies, the inertial force is calculated from three successive inter-particle distances with a certain time interval, in which the inertial force is assumed to be identical at the first two distances. Considering the nature of the attractive force, a new iteration scheme is proposed where the inertial force is assumed to be non-identical from point to point. However, both the identical and non-identical force schemes tend to give an unreasonably oscillating force when the measured inter-particle distance is less accurate. Moreover, the curve fitting function is also considered in this study including the use of polynomial, exponential, sum of sines, rational, Gaussian functions and Fourier series. Among these functions, the 4th order polynomial and the 2nd order sine are the most robust functions in predicting the inertial force on particles, even when the measured distance is less accurate.
View full abstractDownload PDF (1405K) Full view HTML -
Kenso Sugita, Cong Wang, Hiroyuki MatsuuraArticle type: Regular Article
2021 Volume 61 Issue 9 Pages 2410-2415
Published: September 15, 2021
Released on J-STAGE: September 15, 2021
JOURNAL OPEN ACCESS FULL-TEXT HTMLEvolution of TiN inclusion in solid state Fe-based alloy during heating at 1473 and 1673 K was studied by using Fe-36Ni alloy to avoid any unexpected influence of austenite-ferrite phase transformation of alloy on the observation of TiN inclusions in post-annealed samples. Average size of fine TiN inclusions smaller than 2 µm firstly decreased but changed to the increasing tendency during heating at 1473 K, reflecting the initial dissolution of TiN inclusion in Fe-Ni matrix followed by gradual growth of inclusion by Ostwald ripening. On the contrary, heating at 1673 K resulted in the monotonous decrease in average size of TiN inclusion in the size range up to 5 µm. It was considered that above mentioned different TiN inclusion evolution during heating at 1473 or 1673 K was due to the different solubility of TiN depending on temperature; thermodynamic calculation revealed that approximately 40% of TiN inclusion existing at room temperature may dissolve at 1673 K whereas only few may dissolve at 1473 K.
View full abstractDownload PDF (856K) Full view HTML -
Hongying Du, Andrey Vladimirovich Karasev, Pär Göran JönssonArticle type: Regular Article
2021 Volume 61 Issue 9 Pages 2416-2425
Published: September 15, 2021
Released on J-STAGE: September 15, 2021
Advance online publication: March 31, 2021JOURNAL OPEN ACCESS FULL-TEXT HTMLThe focus of this study is to investigate non-metallic inclusions (NMIs) in stainless steels before (in steel samples) and after machining (in steel chips). In this study, the electrolytic extraction (EE) technique was used to extract non-metallic inclusions from steel samples. This makes it possible to investigate NMIs on film filters as three-dimensional objects by using SEM. The characteristics of NMIs in steel and chips have been systematically investigated and compared. Based on the results, it was found that the morphology of NMIs was significantly changed after machining. Overall, three different main shapes of NMIs were found: 1) a similar shape, 2) a stretched shape, and 3) a brittlely fractured shape. Furthermore, the degree of deformation of MnS and soft oxide NMIs in different zones of the chips depends on the distances from the contact zone of the tool and the chip. The total areas of MnS and soft oxides in the secondary deformation zone were increased by up to 2–3 times compared to that of the reference steel sample. This study also shows the advantages of the EE method in investigating NMIs in chips compared to using the conventional two-dimensional investigations of NMIs on the polished metal surface.
View full abstractDownload PDF (1777K) Full view HTML -
Hongying Du, Andrey Vladimirovich Karasev, Pär Göran JönssonArticle type: Regular Article
2021 Volume 61 Issue 9 Pages 2426-2434
Published: September 15, 2021
Released on J-STAGE: September 15, 2021
Advance online publication: June 16, 2021JOURNAL OPEN ACCESS FULL-TEXT HTMLThis research focuses on providing a detailed characteristic of non-metallic inclusions (NMIs) in 316L stainless steels with and without Ca treatment after machining using different cutting speeds. The electrolytic extraction (EE) technique was used for three-dimensional determinations of the inclusion characteristics. Quantitative data from the fragile non-metallic inclusions (such as size, surface area, number) in chips obtained from different cutting speeds and materials were determined. The morphologies of NMIs in the chip samples were quite different compared to the original inclusions in the stainless steel samples before machining. It was proved that the deformation degree of soft inclusions such as MnS and CaO–SiO2–Al2O3–MgO–TiOx is dependent on the cutting speed as well as the temperatures and deformation degrees of the metal matrix during machining. The total surface areas of MnS inclusions increase from 2.8 to 3.8 times compared to the original total areas with an increased cutting speed. The total surface areas of soft oxide inclusions also increase from 1.1 to 3.5 times compared to the original total areas. In addition, the tool-chip contact lengths were also measured on the rake face of the tool, and the results were compared to the determined characteristics of the observed inclusions. It was found that the modification of NMIs by Ca treatment in 316L stainless steels is preferred for high cutting speeds.
View full abstractDownload PDF (1667K) Full view HTML -
Yu Huang, Guoguang Cheng, Meiting Zhu, Shijian Li, Weixing DaiArticle type: Regular Article
2021 Volume 61 Issue 9 Pages 2435-2444
Published: September 15, 2021
Released on J-STAGE: September 15, 2021
Advance online publication: March 11, 2021JOURNAL OPEN ACCESS FULL-TEXT HTMLNiobium (Nb) microalloying can improve the material properties of H13 steel (0.4C-5Cr-1.2Mo-1V steel), but it also affects the natures of the primary carbides. Therefore, the effect of Nb content and cooling rate on the behavior of primary carbides in H13 steel was studied in this paper. The matrix structure was obtained by chemical etching, and then the formation location of primary carbides was identified by electron probe microanalysis (EPMA). The three-dimensional (3D) characteristics, including morphology, number density, and size, were obtained by a non-aqueous electrolysis method. The enrichment of alloying elements in the last-to-solidify region leaded to the formation of primary carbides during the solidification. The Ti4C2S2 phase precipitated first, and then the Mo-Cr-rich carbide was formed around the Ti4C2S2 phase. During the cooling process, the Ti4C2S2 phase partly transformed into Nb-rich carbide and then further partly transformed into V-rich carbide. There is a huge difference between the two-dimensional and three-dimensional morphologies of the primary carbides. As the Nb content increased, the size of last-to-solidify region decreased gradually and the size and number density of primary carbides in the 3D observation increasingly increased. However, as the decrease of the cooling rate, the size of primary carbides increased rapidly and the number density of primary carbides decreased markedly. The thermodynamic and kinetics calculation results agreed well with the experimental observations.
View full abstractDownload PDF (2627K) Full view HTML -
Vipul Kumar Gupta, Pradeep Kumar Jha, Pramod Kumar JainArticle type: Regular Article
2021 Volume 61 Issue 9 Pages 2445-2456
Published: September 15, 2021
Released on J-STAGE: September 15, 2021
JOURNAL OPEN ACCESS FULL-TEXT HTMLErosion of refractory lining due to flow induced wall shear stress is one among severe problems that shop floor personnel face in continuous casting tundish operation. It decreases the lining life and increases overall operational cost. Wall shear stress due to turbulent flow is one of the major factors of erosion of lining in tundish. Inclusion generation due to erosion creates surface defects that may lead to the rejection of the final products. Turbulent inhibitor box (TIB) helps in reducing the wall shear stress by confining the turbulent flow zone and hence changes the flow pattern. In the present work, three-dimensional fluid flow study has been carried out to investigate the flow induced wall shear stress. High shear stress zones (HSSZ) are taken as potential inclusion generation sites. Different sizes of inclusions are injected from those sites and their paths are tracked. It is found that the shape of TIB significantly affects the flow induced wall shear stress and inclusion removal rate. Results indicate that tundish with TIB 3 arrangement exhibited minimum wall shear stress at all walls. TIB 2 coupled tundish gives the highest removal rate in case of bottom wall originated small size inclusions (<= 80 µm) and least removal rate in case of curve wall originated inclusions.
View full abstractDownload PDF (3578K) Full view HTML -
Ming He, Qingwei Wang, Lijia Zhao, Xiaoming Liu, Qiang WangArticle type: Regular Article
2021 Volume 61 Issue 9 Pages 2457-2463
Published: September 15, 2021
Released on J-STAGE: September 15, 2021
JOURNAL OPEN ACCESS FULL-TEXT HTMLOwing to the heat transfer from high-temperature liquid steel, the temperature of the coil in the electromagnetic induction controlled automated steel teeming (EICAST) system in the ladle is very high, seriously restricting the service life of the coil. A novel air-mist cooling method was adopted to reduce the coil temperature in this paper. Firstly, the air-mist generating device was optimized. The results reveal that the optimized air-mist generating device can improve the cooling effect dramatically. And then, the influence of water flow and gas flow on the cooling effect of the coil was analyzed. Both water flow and gas flow affect the final coil temperature and the cooling rate of the coil. The final coil temperature is seen to decrease with an increase in the water flow, and the cooling rate of the coil increases at the initial stage. When the gas flow increases, the final coil temperature does not change much, but the cooling rate of the coil is uncommonly promoted. Finally, a combined cooling method based on the thermal cycle process of the ladle was proposed. After this method, the final coil temperature is 255°C. Compared with air-mist cooling, the final temperature difference is not large, but the coil temperature is kept in a low level for a long time by using the combined cooling. These studies will promote the application of the EICAST technology which can reduce non-metallic inclusions in steels.
View full abstractDownload PDF (1105K) Full view HTML -
Hae-Mi Hong, Youn-Bae KangArticle type: Regular Article
2021 Volume 61 Issue 9 Pages 2464-2473
Published: September 15, 2021
Released on J-STAGE: September 15, 2021
Advance online publication: March 19, 2021JOURNAL OPEN ACCESS FULL-TEXT HTMLIn order to evaluate cleanliness of steel samples which contain oxygen in two different forms - chemically dissolved form and physically dispersed form in steel matrix, identification of each oxygen in the steel is important. A simple but promising method for simultaneous analysis of these two types oxygen in steel samples was developed in the present study using Inert Gas Fusion Infrared Absorption Method. By utilizing different carbothermic reaction temperature for each type of oxygen, the chemically dissolved oxygen (soluble oxygen) was first separated from the steel specimen at a low reaction temperature, while the physically dispersed oxygen (insoluble oxygen in the form of oxide inclusion) was separated at a higher reaction temperature. This idea was applied to a number of Al-killed ultra low carbon steel specimens, which contain alumina inclusions. It was shown that separation of the soluble oxygen and the insoluble oxygen was possible. The obtained oxygen content in this new method was independently validated by a conventional Inert Gas Fusion Infrared Absorption Method for the total oxygen content and by cross-sectional analysis of non-metallic inclusion for the insoluble oxygen. A possible supersaturation state of liquid steel after RH process was observed.
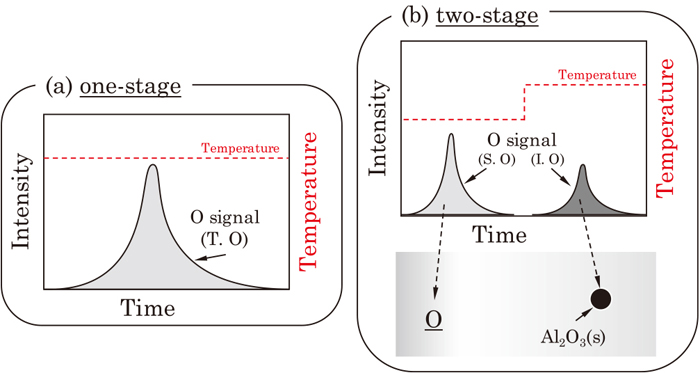 Signal of CO gas evolution from a specimen to analyze the O content (not to scale): (a) conventional heating pattern (one-stage) (b) two-stage heating pattern proposed in the present study. (Online version in color.) Fullsize ImageView full abstractDownload PDF (765K) Full view HTML
Signal of CO gas evolution from a specimen to analyze the O content (not to scale): (a) conventional heating pattern (one-stage) (b) two-stage heating pattern proposed in the present study. (Online version in color.) Fullsize ImageView full abstractDownload PDF (765K) Full view HTML
- |<
- <
- 1
- >
- >|