All issues

Volume 56, Issue 2
Displaying 1-25 of 25 articles from this issue
- |<
- <
- 1
- >
- >|
Fundamentals of High Temperature Processes
Regular Article
-
Bora Derin, Emre Alan, Masanori Suzuki, Toshihiro TanakaArticle type: Regular Article
2016 Volume 56 Issue 2 Pages 183-188
Published: February 15, 2016
Released on J-STAGE: March 04, 2016
JOURNAL OPEN ACCESS FULL-TEXT HTMLIn the present study, the impurity capacities (Ci) of phosphate, phosphide, nitride and carbide in binary and multi-component molten melt systems at different temperatures were estimated using the artificial neural network approach. The experimental data taken from the previous studies were introduced to the artificial neural network, then the calculated results were plotted against the experimental values for comparative purposes. Besides, iso-phosphate capacity contours on the liquid region of CaO–CaF2–Al2O3 ternary phase diagram at 1773 K were generated and plotted by using the neural network model results. The calculated results obtained through neural network computation agreed well with the experimental ones and were found more accurate than those estimates based on some models.View full abstractDownload PDF (807K) Full view HTML -
Bin Li, Junhong Chen, Mingwei Yan, Jindong Su, Jialin Sun, Yong LiArticle type: Regular Article
2016 Volume 56 Issue 2 Pages 189-194
Published: February 15, 2016
Released on J-STAGE: March 04, 2016
Advance online publication: December 25, 2015JOURNAL OPEN ACCESS FULL-TEXT HTMLTo study the substitution of Fe3Si–Si3N4 for refractories in the upper RH refiner, this paper simulated the service condition of RH refining and studied the change of the Fe3Si–Si3N4 in the simulated condition. A Fe3Si–Si3N4 specimen prepared by flash combustion was put in a vacuum sintering furnace with carbon lining, fired at 1450°C under 80 Pa of vacuum degree for 2 h, and then cooled. The morphological evolution before and after being treated and phase interactions of the Fe3Si–Si3N4 specimen were studied and analyzed thermodynamically and dynamically. The results show that at high temperatures in vacuum, Fe volatilizes from the Fe3Si melt in Fe3Si–Si3N4 and reacts with Si3N4 on the Si3N4 crystal surface, forming new FexSi melt there; then Fe continues to volatilize from the new FexSi melt, causing FexSi alloy particles finer and more uniform in Fe3Si–Si3N4; the hexagonal columnar Si3N4 crystals begin to decompose partially, and become cylindrical with edges and corners disappearing; during prebaking or operation interval of RH refining, a SiO2 film which has better stability than Si3N4 is developed on the surface of Si3N4 crystals or Fe3Si–Si3N4 bricks, preventing the decomposition of Si3N4 and improving the application feasibility of Fe3Si–Si3N4 in RH refining.View full abstractDownload PDF (763K) Full view HTML -
Xiao-Hong Tian, Wan-Yuan Shi, Tian Tang, Lin FengArticle type: Regular Article
2016 Volume 56 Issue 2 Pages 195-204
Published: February 15, 2016
Released on J-STAGE: March 04, 2016
Advance online publication: December 25, 2015JOURNAL OPEN ACCESS FULL-TEXT HTMLIn order to investigate the influence of static magnetic field on behavior of rising single bubble in conductive fluid, a series of axisymmetric numerical simulations are carried out. A uniform vertical magnetic field with intensities ranging from 0 to 0.4 T (Ha=0−16.97) is superposed and the bubble radii range from 2 mm to 6 mm (R*=0.2−0.6). The rising velocity, instantaneous bubble shape and terminal height are discussed and whether the magnetic field restrains bubble rising simply or transits from positive effect to negative one with the increasing of magnetic field intensities is analyzed clearly. Besides, the discrepancy of bubble motion under magnetic field with weak or strong surface tension is compared. Numerical results show that vertical magnetic field elongates the bubble shape along vertical direction and a stronger magnetic field intensity contributes to a longer bubble shape. The imposed magnetic field has an inhibitory effect on the rising velocity for bubbles with strong surface tension as well as bubbles with weak surface tension but small sizes. However, for bubbles with weak surface tension but large sizes, the rising velocity is promoted by weak magnetic field intensities whereas inhibited by strong magnetic field intensities. The peak Hartmann number reaching the strongest positive effect and the critical Hartmann number turning from positive effect to negative effect are determined respectively. View full abstractDownload PDF (1510K) Full view HTML
View full abstractDownload PDF (1510K) Full view HTML -
Wanli Li, Xiaozhou Cao, Tao Jiang, He Yang, Xiangxin XueArticle type: Regular Article
2016 Volume 56 Issue 2 Pages 205-209
Published: February 15, 2016
Released on J-STAGE: March 04, 2016
JOURNAL OPEN ACCESS FULL-TEXT HTMLAlternating current impedance method has been used to measure the electrical conductivity of CaO–SiO2–Al2O3–MgO system and viscosity of this system has been measured by high temperature rheometer at the same time. It is found that measured data of electrical conductivity and viscosity showed a relatively high accuracy. A relation between the two parameters has been estimated using a formula which showed a relatively lower deviation compared to the precious work. Ionization theory of molten slag has been used to explicate the electrical conductivity and viscosity of molten slag. Schematic illustration of experimental apparatus. Fullsize ImageView full abstractDownload PDF (1000K) Full view HTML
Schematic illustration of experimental apparatus. Fullsize ImageView full abstractDownload PDF (1000K) Full view HTML
Ironmaking
Regular Article
-
Jue Tang, Mansheng Chu, Cong Feng, Yating Tang, Zhenggen LiuArticle type: Regular Article
2016 Volume 56 Issue 2 Pages 210-219
Published: February 15, 2016
Released on J-STAGE: March 04, 2016
JOURNAL OPEN ACCESS FULL-TEXT HTMLThe recoveries of vanadium, chromium, and titanium are much low in the current process of high-chromium vanadium–bearing titanomagnetite. The gas-based direct reduction followed by melting separation process was proposed because the valuable elements including iron, vanadium, chromium, and titanium can be utilized simultaneously and all the recoveries should increase. The melting separation study was carried out in the present work, and the behavior as well as mechanism during melting separation process was investigated. The effect of temperature on the melting separation kinetic was more considerable than thermodynamic. As increased basicity, the area of liquid phase was enlarged and the solubility of Al2O3 in slag was increased. The main mineral in separation slag was anosovite (MgTi2O5), besides a small number of pseudobrookite (Fe2TiO5), perovskite (CaTiO3), and spinel (Ca3Al2O6). The melting separation was improved by the increasing C/O, temperature, time, additive of CaF2, and basicity. The rational melting separation parameters for high-chromium vanadium–bearing titanomagnetite contained a C/O of 1.2, a melting temperature of 1625°C, a melting time range of 40–50 min, an additive CaF2 of 2%, and a basicity of 1.1. Under these conditions (melting time of 50 min), the melting separation was achieved successfully. Both the titanium-bearing slag together with the iron containing vanadium and chromium was obtained. The recoveries of Fe, V, Cr, and TiO2 could reach around 99%, 97%, 92%, and 94%, respectively; and the mass fraction of Fe, V, Cr, and TiO2 were 93.50%, 0.90%, 0.69%, and 37.52%, respectively.View full abstractDownload PDF (1991K) Full view HTML -
Miyuki Hayashi, Kyohei Suzuki, Yasuhiro Maeda, Takashi WatanabeArticle type: Regular Article
2016 Volume 56 Issue 2 Pages 220-225
Published: February 15, 2016
Released on J-STAGE: March 04, 2016
Advance online publication: January 14, 2016JOURNAL OPEN ACCESS FULL-TEXT HTMLTo investigate the relation between Al2O3 and the gas permeability resistance in a blast furnace, the effect of Al2O3 on the increment in the slag melt volume and the decrement in the temperature of the slag melt formation has been elucidated by comparing the reactions between liquid olivine and solid 2CaO∙SiO2 and between liquid olivine and solid 2CaO∙Al2O3∙SiO2 at the constant temperature of 1240°C, and eutectic reactions between FeO and 2CaO∙SiO2 and between FeO and 2CaO∙Al2O3∙SiO2 at the constant heating rate of 10°C/min. The reaction temperature of 1240°C has been chosen because the effect of Al2O3 on the gas permeability is markedly observed at that temperature. The heating rate has been set to be 10°C/min as it is considered to be the approximate heating rate of burden in a blast furnace. The fraction of the liquid phase decreases from 38.3% to 30% for olivine-2CaO∙SiO2 system, while it increases from 23.5% to 27.4% for olivine-2CaO∙Al2O3∙SiO2 system in 10 min after the onset of heating. It is considered that the increment in the Al2O3 content of iron ores raises the amount of olivine-like slag melts around 1240°C. The onset temperature of eutectic reaction for mixed FeO and C2AS powders is almost equal to that for mixed FeO and C2S powders when the temperatures are compared for powders with the average particle size smaller than around 40 µm. This implies that a negative effect of Al2O3, that is, lowering the temperatures of slag melt formation could be eliminated when the microstructures of sinters are fine enough.View full abstractDownload PDF (1176K) Full view HTML -
Jin-Young An, Jong-Beom Seo, Ju-Hee Choi, Jong-Hyup Lee, Hyuk KimArticle type: Regular Article
2016 Volume 56 Issue 2 Pages 226-232
Published: February 15, 2016
Released on J-STAGE: March 04, 2016
Advance online publication: December 25, 2015JOURNAL OPEN ACCESS FULL-TEXT HTMLIn this study, post reaction strength under various conditions was measured to investigate effects of gasification with CO2 on coke degradation in the lower shaft of blast furnace. Post reaction strength of coke samples taken at the shaft of actual blast furnace was much higher than that in NSC tests, leading to slight change in mean size. The test for coke strength after reaction with the constant weight loss of 20% was not an effective way to evaluate commecial coke with high CSR and low CRI. When coke samples were gasified at a different temperature of 1100–1300°C, topochemical reacton was observed at over 1200°C by image analysis. Post reaction strength (CSRSBF) in the simulated blast furnace conditions was 14.9%P higher than conventional CSRs, which is attributed to different correlationship between reactivity and post reaction strength in two methods. This phenomenon seems to be caused by topochemical reaction on coke surface in blast furnaces which results into suppressing coke degradation. The post reaction strength test should be modified in accordance with the individual blast furnace operation, to simulate coke degradation due to solution loss in the shaft because solution loss in NSC tests is overestimated.View full abstractDownload PDF (890K) Full view HTML -
Chishiro Funada, Taichi Murakami, Eiki KasaiArticle type: Regular Article
2016 Volume 56 Issue 2 Pages 233-238
Published: February 15, 2016
Released on J-STAGE: March 04, 2016
JOURNAL OPEN ACCESS FULL-TEXT HTMLSince iron ore-carbon composite permits rapid reduction reaction, its utilization is an effective method to reduce carbon dioxide emissions in ironmaking process. Major iron ores consist of Fe2O3, Fe3O4, and FeOOH, which require several reduction steps through FexO to form metallic iron. In this study, FexO, which has a certain solid solution range, was highlighted as an alternative iron source for the composite. It is because FexO can be produced by a pre-reduction process of iron ores although it is not a major component of natural ores. The effect of the oxygen concentration of the FexO and the mix-grinding time of the iron oxide and carbonaceous material on the reduction behavior of the composite was examined.
The composite samples of FexO with different oxygen concentrations, i.e., different x value and graphite were prepared and heated up to 1200°C under inert gas flow. The value of reduction degree was calculated from the concentration of gas generated from the composite. Reduction temperature decreases with increasing x value and the grinding time. In the case of a 3.6 ks-grinding, reduction reaction of the composite with a low x value proceeded homogeneously, while reduction of that with a high x value proceeded topochemically.View full abstractDownload PDF (1012K) Full view HTML -
Yuki Iwai, Natsuo Ishiwata, Ryota Murai, Hidetoshi MatsunoArticle type: Regular Article
2016 Volume 56 Issue 2 Pages 239-244
Published: February 15, 2016
Released on J-STAGE: March 04, 2016
JOURNAL OPEN ACCESS FULL-TEXT HTMLShaft furnace (cupola) is a scrap melting furnace and it can reduce CO2 emission because it requires no reducing agents. In a shaft furnace, coke gasification reactions by CO2 and H2O are occurred in high temperature and they have great effects on coke rate in shaft furnace because they are strong endothermic reactions. So prediction of shaft furnace operation needs accurate gasification rates. In this study, first, gasification rates by CO2/N2 and H2O/N2 system were measured at 1573, 1673, 1773 and 1873 K. Diffusion coefficients in gasification rates were modified to fit experimental results. Next, one-dimensional mathematical model using modified gasification rates was developed for shaft furnace simulation. The effects of coke size and blast moisture on coke rate were calculated and compared with actual operations. Operational results were accurately predicted by the model.View full abstractDownload PDF (1757K) Full view HTML -
Heng Zhou, Zhi-Guo Luo, Tao Zhang, Yang You, Zong-Shu Zou, Yansong She ...Article type: Regular Article
2016 Volume 56 Issue 2 Pages 245-254
Published: February 15, 2016
Released on J-STAGE: March 04, 2016
JOURNAL OPEN ACCESS FULL-TEXT HTMLIn this work, a three-dimensional DEM-based model is used to investigate solid flow including burden descending behaviour and particle segregation in the shaft furnace of ironmaking COREX. The model was validated by the reasonable agreements between simulations and experiments. The results confirm that the Areal Gas Distribution (AGD) beams affect solid flow in the bustle zone significantly but slightly at the lower part of the furnace. A triangle-shaped free zone is observed below the AGD beams, which will be the main path for gas flow along the centre of shaft furnace. The AGD beams can lead to particle segregation in the shaft furnace. The coke and flux particles can roll easily from the centre to the zone below the AGD. As a result, the normalized mass fractions of coke and flux are larger than 1.0 near the screw outlet located below the AGD beams. In addition, three new designs of AGD beams are proposed in this work. The simulation results indicate that the design with three AGD beams is regarded as the optimal option due to uniform burden descent and convenient installation. The model provides a cost-effective tool to understand and optimize the solid flow in the shaft furnace of COREX.View full abstractDownload PDF (1790K) Full view HTML
Steelmaking
Regular Article
-
Huai Wang, Yunbo Zhong, Qiang Li, Yipeng Fang, Weili Ren, Zuosheng Lei ...Article type: Regular Article
2016 Volume 56 Issue 2 Pages 255-263
Published: February 15, 2016
Released on J-STAGE: March 04, 2016
Advance online publication: January 14, 2016JOURNAL OPEN ACCESS FULL-TEXT HTMLA transparent experimental model had been built to visualize the electroslag remelting (ESR) process. With the help of the transparent model, the evolution of the droplet happening at the tip of the consumable electrode was recorded clearly by a high-speed camera with the record frequency of 200 frames per second. Firstly, the physical simulations were done under different intensities of the transverse static magnetic field (TSMF) with a constant remelting current of 8 A. Then the experiments were carried out at different intensities of the remelting current with a constant TSMF of 0.7 T. The representative processes of the droplet evolution under different conditions were demonstrated. When the external TSMF was large enough, a special phenomenon was discovered: The liquid neck connecting the end of the electrode with the droplet would become longer, and then an inflated liquid bulge appeared in the middle of the liquid neck. After a short while, the liquid metal neck was smashed into a lot of smaller droplets by the strong electromagnetic vibration. The results under different conditions indicated that the smashing effect acting on the droplet neck would be improved with the enlarging of the external TSMF at the constant remelting current, but it got worse under the condition of a higher remelting current. The mechanism of the smashing effect was well discussed. The representative droplet evolution conducted under the condition of the remelting current of 8 A and the TSMF of 0.7 T. Fullsize ImageView full abstractDownload PDF (4249K) Full view HTML
The representative droplet evolution conducted under the condition of the remelting current of 8 A and the TSMF of 0.7 T. Fullsize ImageView full abstractDownload PDF (4249K) Full view HTML
Casting and Solidification
Regular Article
-
Kenneth C. Mills, Shyamprasad Karagadde, Peter David Lee, Lang Yuan, F ...Article type: Regular Article
2016 Volume 56 Issue 2 Pages 264-273
Published: February 15, 2016
Released on J-STAGE: March 04, 2016
Advance online publication: January 14, 2016JOURNAL OPEN ACCESS FULL-TEXT HTMLPhysical properties of both steels and mould slags are needed as input data for the mathematical modelling of the continuous casting process. Routines for calculating the properties of mould slags and for estimating steel properties have been developed and are described in Parts 1 and 2, respectively. Many mould powders, with differing compositions, are used in casting practice and their properties vary significantly. Reliable models have been developed to calculate these property values as a function of temperature from their chemical composition since this is available on a routine basis. Models have been developed to calculate the following properties: heat capacities, enthalpies, thermal expansion coefficient, density, viscosity, thermal conductivity and surface tension. Solid mould slags can exist as glassy or crystalline phases or as mixtures of the two (i.e. slag films) and the properties for the various phases can vary considerably; methods have been developed to calculate property values for these various states. The software used to calculate the properties is available via the link (i) http://www.mxif.manchester.ac.uk/resources/software (ii) https://sites.google.com/site/shyamkaragadde/software/thermophysical-properties.View full abstractDownload PDF (602K) Full view HTML -
Kenneth C. Mills, Shyamprasad Karagadde, Peter David Lee, Lang Yuan, F ...Article type: Regular Article
2016 Volume 56 Issue 2 Pages 274-281
Published: February 15, 2016
Released on J-STAGE: March 04, 2016
Advance online publication: January 14, 2016JOURNAL OPEN ACCESS FULL-TEXT HTMLThe objective of the present study was to calculate physical property values for steels from their chemical compositions for subsequent use in mathematical models of the fluid flow, heat transfer and shell solidification in the continuous casting mould. Values of the following properties of steels are calculated for temperatures between 298 K and 2000 K; Heat Capacity (Cp) Density (ρ) Thermal conductivity (k) and diffusivity (a) Electrical resistivity (R) Viscosity (η) Surface (γm) and Interfacial tension (γmsl). In addition temperatures of transitions (Liquidus Tliq, Solidus Tsol) and various solid state transitions were also calculated. Ferritic and austenitic phases of Carbon - and stainless steels are both covered. The associated software is available on the following websites (i) http://www.mxif.manchester.ac.uk/resources/software (ii) https://sites.google.com/site/shyamkaragadde/software/thermophysical-properties.View full abstractDownload PDF (980K) Full view HTML -
Qiang Wang, Baokuan LiArticle type: Regular Article
2016 Volume 56 Issue 2 Pages 282-287
Published: February 15, 2016
Released on J-STAGE: March 04, 2016
JOURNAL OPEN ACCESS FULL-TEXT HTMLA transient three-dimensional (3D) model was developed to understand the role slag thickness plays in the formation of the metal pool in the electroslag remelting (ESR) process. In this model, the solution of the mass, momentum and energy conservation equations were simultaneously implemented by the finite volume method with full coupling of the Joule heating and Lorentz force by solving the Maxwell’s equations. The movement of metal droplet was described by volume of fluid (VOF) approach. Additionally, the solidification was modeled using an enthalpy-based technique, where the mushy zone was treated as a porous medium. The experiment and simulation demonstrated a reasonable agreement. The results indicate that changing the slag thickness changes the slag temperature, but not monotonically. The slag temperature drops with the slag thickness up to 60 mm, beyond which the slag temperature rises. The melt rate decreases and then increases while the cooling intensity remains unchanged. As a consequence, the maximal metal pool depth reduces from 0.081 m to 0.067 m and slightly increases to 0.074 m.View full abstractDownload PDF (1815K) Full view HTML
Instrumentation, Control and System Engineering
Regular Article
-
QingXue Huang, Jia Li, HongZhou Li, HeYong Han, LiDong MaArticle type: Regular Article
2016 Volume 56 Issue 2 Pages 288-293
Published: February 15, 2016
Released on J-STAGE: March 04, 2016
JOURNAL OPEN ACCESS FULL-TEXT HTMLIn this study, the model of new hydraulic bilateral rolling shear is researched based on kinematics theory of a hybrid spatial linkage mechanism. Combined with research method of 3-RRR planar parallel mechanisms kinematic, the relationship between cylinder displacements and blade positions of the shearing mechanism is developed. Nonlinear system stability of the shearing mechanism in a new hydraulic bilateral rolling shear is researched based on Krasovskii method, the result shown that the system is stable and reliable. Moreover, based on the bilateral rolling shear of a large steel company, the displacement of cylinders is obtained through experiments. Compared with differential between the theoretical formula and the actual value, the differential is small. This study has certain instruction to the design and the development of similar product and it also has a higher practical value to the general industrial production.View full abstractDownload PDF (1011K) Full view HTML
Forming Processing and Thermomechanical Treatment
Regular Article
-
Naoki Nakata, Takashi Kuroki, Akio Fujibayashi, Yoshimichi Hino, Yoshi ...Article type: Regular Article
2016 Volume 56 Issue 2 Pages 294-302
Published: February 15, 2016
Released on J-STAGE: March 04, 2016
JOURNAL OPEN ACCESS FULL-TEXT HTMLLaboratory tests were conducted in order to quantify the cooling performance of intensive inter-pass water cooling, which was introduced as an effective method for increasing productivity of high strength steel plates.
The range of flow rates was extended to 0.17–0.39 m3/m2 s. Pipe nozzles with inner diameters of 3 mm or 6 mm were used in addition to the original hole-type nozzle. The effects of the type of nozzle, the density of the nozzles and the injection distance on cooling performance were investigated. In tests with the ϕ6 pipe nozzles, temperature drop in the specimens increased with higher water flow rates. The upper limit of cooling performance was found to be around 0.25 m3/m2 s in top side cooling, whereas an upper limit was not seen in bottom side cooling. In tests with the ϕ3 hole nozzles, temperature drop decreased with longer injection distance, and that tendency is larger in bottom side cooling. A nozzle arrangement with a shorter installation pitch results in higher cooling performance. The effect of the inner diameter of the pipe nozzles on cooling performance is small.
After water cooling, controlled rolling of specimens of Si–Mn–C steel, which is used widely as a high tensile steel, was performed with a laboratory mill. As results, it was found that total rolling time can be reduced with inter-pass water cooling, and water cooling does not affect the microstructure or mechanical properties.
Intensive inter-pass cooling, which has high cooling performance, has the potential to realize efficient production of high-strength steels in controlled rolling.View full abstractDownload PDF (981K) Full view HTML -
Nao-Aki Noda, Yuanming Xu, Dedi Suryadi, Yoshikazu Sano, Yasushi Takas ...Article type: Regular Article
2016 Volume 56 Issue 2 Pages 303-310
Published: February 15, 2016
Released on J-STAGE: March 04, 2016
JOURNAL OPEN ACCESS FULL-TEXT HTMLCeramic rollers can be used in the heating furnace conveniently because of its high temperature resistance. The new roller consists of ceramic sleeve and steel shaft connected only under a small shrink fitting ratio because of the brittleness. In the previous study, simulation of coming out of the shaft from the sleeve was performed, but only small number of loading cycle can be considered because of large computational time. To analyze the coming out problem more efficiently, in this paper, the two-dimensional shrink fitted structures are considered. Then, the effects of the magnitude of load and shrink fitting ratio are investigated under large number of loading cycle. Finally, the coming out mechanism is discussed for larger number of cycles by focusing on the shear stress and displacement at the joint end. The coming out occurs when the negative shear stress is unstable. On the other hand, the coming out does not occur when the positive shear stress is stable with little fluctuation.View full abstractDownload PDF (2079K) Full view HTML
Transformations and Microstructures
Regular Article
-
Noriaki Kosaka, Yoshimasa FunakawaArticle type: Regular Article
2016 Volume 56 Issue 2 Pages 311-318
Published: February 15, 2016
Released on J-STAGE: March 04, 2016
JOURNAL OPEN ACCESS FULL-TEXT HTMLWork hardening of ferritic steels containing fine carbides varied from 3 nm to 15 nm was investigated and compared to Ashby’s model, which is well known as a work hardening theory of metals containing hard particles. A specific work hardening behavior was observed in the steels strengthened by the nanometer-sized carbides; work hardening proceeded in two stages within a few plastic strains. In the former step, the matrix deformed without the geometrically necessary dislocation since a misfit strain between the carbides and matrix is close to the Burgers vector. So the Ashby’s model cannot explain this phenomenon. Yet in the later stage, the amount of work hardening was close to predicted value based on the Ashby’s model. The plastic strain at which the later stage started decreased with the increase in the diameter of carbides since the geometrically necessary dislocation is easier to be generated by the larger carbides. A new model which can be applied to steels containing the nanometer-sized carbides by focusing generating dislocation into the matrix around carbides was established.View full abstractDownload PDF (963K) Full view HTML -
Naoki Takata, Tadashi Tsukahara, Satoru Kobayashi, Masao TakeyamaArticle type: Regular Article
2016 Volume 56 Issue 2 Pages 319-325
Published: February 15, 2016
Released on J-STAGE: March 04, 2016
JOURNAL OPEN ACCESS FULL-TEXT HTMLThis study investigates the γ→α transformation kinetics of Fe–Mn–C dual-phase steels at a temperature above the melting point of an Al–8.2Mg–4.8Si (wt.%) alloy coating formed on their surface by hot-dipping process. Using an experimentally determined time-temperature-transformation (TTT) diagram for a model steel of Fe–1.5Mn–0.1C (wt.%), the volume fraction of martensite is controlled through the intercritical heat treatment incorporated into the hot-dipping process. The microstructural observations confirm that this combined process route makes it possible to fabricate hot-dipped Al alloy-coated dual-phase steels with a controlled microstructure.View full abstractDownload PDF (1797K) Full view HTML -
Shu-Yu Wu, Chun-Hung Lin, Wei-Chih Hsu, Liuwen Chang, Pei-Ling Sun, Po ...Article type: Regular Article
2016 Volume 56 Issue 2 Pages 326-334
Published: February 15, 2016
Released on J-STAGE: March 04, 2016
Advance online publication: January 14, 2016JOURNAL OPEN ACCESS FULL-TEXT HTMLThe heating rate effect on texture evolution of a 1.09 wt.% Si non-oriented electrical steel was studied by using two heating rates, 0.5°C/s (slow heating) and 15°C/s (fast heating), in the final annealing. The comparison of textures was made on similar microstructural basis, i.e., recrystallized fraction in the recrystallization stage and grain size in the grain growth stage. Change of heating rate caused little influence on the texture evolution in the recrystallization stage, implying that the texture at complete recrystallization is mainly determined by the cold-rolling structure. In the growth stage, the heating rate caused little influence on the evolution of {111}<110>, {111}<112>, and Cube components, however, made significant influence on the evolution of Goss component. For both heating rates, the intensity of Goss component increased continuously during recrystallization, but decreased rapidly as the grains grew. Comparing to the fast heating, the slow heating caused more drastic decrease in Goss component as the grains grew. Microstructure observations indicated that the average size of Goss grains was similar to that of the average recrystallized grains in the recrystallization stage, but became smaller than that of the average grains in the growth stage. It was attributed to an orientation pinning effect due to the preferential nucleation of Goss grains at shear bands. Rapid heating could make the Goss grains more dispersed in the recrystallized structure, which reduced the probability of orientation pinning encountered by these Goss grains, and delayed the decrease of growth rate of Goss grains in grain growth.View full abstractDownload PDF (1736K) Full view HTML -
Kuo-Tsung Huang, Shih-Hsien Chang, Ming-Wei Wu, Chih-Kai WangArticle type: Regular Article
2016 Volume 56 Issue 2 Pages 335-340
Published: February 15, 2016
Released on J-STAGE: March 04, 2016
JOURNAL OPEN ACCESS FULL-TEXT HTMLThis study utilized two different sizes of 440C pre-alloy powders for vacuum sintering under different sintering temperatures (1523, 1533, 1543 and 1553 K) to explore the microstructure and mechanical properties of 440C stainless steel. The experimental results indicated that the sintered specimens of the refined alloy powders had good hardness and transverse rupture strength (TRS), due to the smaller grain size of the sintered body and the uniform distribution of the carbides. In addition, two types of carbide precipitates appeared in the microstructure. Fine carbides (M23C6) within the grains and rod-shaped types of carbide (M7C3) on the grain boundaries were observed after sintering at 1543 K for 1 h. The research also showed that a suitable amount of carbides is helpful in improving the TRS. However, the fine carbides (M23C6) within the grains almost disappeared and the rod-shaped types of carbide (M7C3) on the grain boundaries coarsen after sintering at higher temperatures (1553 K), and so detrimental to the TRS.View full abstractDownload PDF (1426K) Full view HTML
Mechanical Properties
Regular Article
-
Kazuki Shibanuma, Yuki Yamamoto, Fuminori Yanagimoto, Katsuyuki Suzuki ...Article type: Regular Article
2016 Volume 56 Issue 2 Pages 341-349
Published: February 15, 2016
Released on J-STAGE: March 04, 2016
JOURNAL OPEN ACCESS FULL-TEXT HTMLA new multiscale model is proposed by a “model synthesis” approach, as the first attempt to clarify the relationship between microstructures of steel and macroscopic brittle crack propagation and arrest behavior. The first part of the present paper shows the model presentation. The multiscale model consists of two models: (1) a microscopic model to simulate cleavage fracture in the grain scale and (2) a macroscopic model to simulate brittle crack propagation and arrest behavior in the steel plate scale. In both the models, we utilize the same framework, where a simple two-dimensional domain discretization is employed but a three-dimensional crack propagation can be effectively modeled. The discretized unit cells in the microscopic model correspond to the grain size. On the other hand, the discretized unit cells in the macroscopic model correspond to the entire domain of the microscopic model. The microscopic model proposed by Aihara and Tanaka is basically employed except the integration with the macroscopic model. The effective surface energy, which is used for the integration between microscopic and macroscopic models, is assumed as the plastic work to form tear-ridge. The proposed model synthesis for multiscale model as an integrated macroscopic model is performed by systematically incorporating (1) the preparatory macroscopic finite element analysis and (2) the Monte Carlo simulation of microscopic analysis into (3) the macroscopic analysis for brittle crack propagation and arrest in steel plate. The integration procedure is implemented by the assignment of physical quantities based on interpolation methods as a one-way coupling algorithm for simplification. Procedure of the integrated macroscopic model by model synthesis in the multiscale model. (Online version in color.) Fullsize ImageView full abstractDownload PDF (1118K) Full view HTML
Procedure of the integrated macroscopic model by model synthesis in the multiscale model. (Online version in color.) Fullsize ImageView full abstractDownload PDF (1118K) Full view HTML -
Yuki Yamamoto, Kazuki Shibanuma, Fuminori Yanagimoto, Katsuyuki Suzuki ...Article type: Regular Article
2016 Volume 56 Issue 2 Pages 350-358
Published: February 15, 2016
Released on J-STAGE: March 04, 2016
JOURNAL OPEN ACCESS FULL-TEXT HTMLThe second part of the present paper shows an application of the proposed multiscale model to the temperature gradient crack arrest test of the steel plates having nonhomogeneous distributions of microstructures in thickness direction. The multiscale model is developed as the integrated macroscopic model composed of the three-staged analyses. The first stage is a preparatory macroscopic finite element analysis, where the nodal force release method is employed to simulate fast crack propagation under the dynamic elastic-plastic condition without considering non-linearity of geometry. The second stage is the Monte Carlo simulation of microscopic analysis for cleavage fracture at the discrete evaluation points. The results of local fracture toughness and direction of fracture surface show large scatters even at the same evaluation point. The final stage is the integrated macroscopic analysis, which is composed of the two parts: (a) assignment of parameters obtained in the previous analyses in each unit cell, and (b) simulation of brittle crack propagation/arrest behavior. As a result, the proposed multiscale model successfully simulated the complicated brittle crack propagation/arrest behavior. In particular, not only the arrested crack length but also the characteristic fracture surface such as “split nails” were accurately simulated. It is therefore found that the proposed model has been validated by the comparison with experiment. That is, the proposed model in the present study has a potential basis of the framework to establish the theory for the clarification of the relationship between microstructures of steel and macroscopic arrest toughness of steel plate.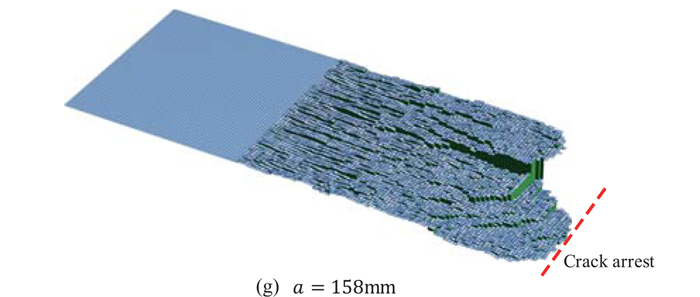 Simulation results of fracture surface. (Online version in color.) Fullsize ImageView full abstractDownload PDF (1576K) Full view HTML
Simulation results of fracture surface. (Online version in color.) Fullsize ImageView full abstractDownload PDF (1576K) Full view HTML -
Daisuke Hirakami, Shingo Yamasaki, Toshimi Tarui, Kohsaku UshiodaArticle type: Regular Article
2016 Volume 56 Issue 2 Pages 359-365
Published: February 15, 2016
Released on J-STAGE: March 04, 2016
JOURNAL OPEN ACCESS FULL-TEXT HTMLHydrogen embrittlement has become a crucial issue with the promotion of high-strength steel. Many studies have been conducted on the mechanism of hydrogen embrittlement. Because the elucidation of the state of hydrogen is important to understand the mechanism, the states of hydrogen in the steels investigated were controlled. In the present study, 0.35 mass% C and 0.8 mass% C steels annealed in the hydrogen atmosphere followed by quenching from the austenite region together with drawn pearlitic steel of 0.8 mass% C were used to analyze the state of the hydrogen contributing to the emission peak, in particular, at about 300°C in the Thermal Desorption Analysis (TDA) curve. The peak at 300°C was significant for quenched 0.8 mass% C steel with low Ms temperature; however, the peak decreased with aging at room temperature. However, in 0.35 mass% C steel with high Ms temperature, the peak at 300°C was no longer observed. Moreover, in the hydrogen charged as drawn 0.8 mass% pearlitic steel, the peak at 300°C did not change with aging at room temperature because of no significant carbon in solid solution, while the peak at 100°C decreased with the increase in aging time. Taking into account the competitive phenomenon of hydrogen trapping at the dislocation core and C segregation to dislocations during room temperature aging or during quenching from Ms temperature, it was concluded that the hydrogen peak at about 300°C is hydrogen trapped in the dislocation core, while the other hydrogen peak at 100°C is attributed to the hydrogen trapped by the stress field generated by dislocation.View full abstractDownload PDF (1095K) Full view HTML
Physical Properties
Regular Article
-
Mu Li, Rie Endo, Li Ju Wang, Lei Li, Masahiro SusaArticle type: Regular Article
2016 Volume 56 Issue 2 Pages 366-375
Published: February 15, 2016
Released on J-STAGE: March 04, 2016
JOURNAL OPEN ACCESS FULL-TEXT HTMLA new apparatus to measure a heat flux across a sheet sample has been proposed to determine its apparent thermal conductivity. The heat flux was derived from the volume change caused by melting of ice utilizing the principle of the Bunsen Ice Calorimeter. Measurements were conducted using Ni-base super alloy (Inconel 600), alumina and Teflon as samples to confirm the reasonability of this method, in addition to a simulation study. The apparent thermal conductivity values obtained are 14.7±0.4 Wm−1K−1 for Inconel at 281 K–287 K, 24.8±0.7 Wm−1K−1 for alumina at 281 K–287 K and 0.313±0.004 Wm−1K−1 for Teflon at 285 K–411 K, which are in very good agreement with the respective reported values. Discussion has been made on error factors of this method and a feasibility of this method being applied to measurements of apparent thermal conductivities of oxide scales formed on the steel surface under steep temperature gradient in the actual hot-rolling process.View full abstractDownload PDF (1171K) Full view HTML
- |<
- <
- 1
- >
- >|