
- Issue 4 Pages 267-
- Issue 3 Pages 185-
- Issue 2 Pages 113-
- Issue 1 Pages 1-
- |<
- <
- 1
- >
- >|
-
Risei WADA, Ryoichi YOSHIDA, Kazumasa SUZUKI, Hidenobu SHIMIZU, Masaru ...2019 Volume 76 Issue 4 Pages 267-275
Published: July 25, 2019
Released on J-STAGE: July 25, 2019
Advance online publication: May 24, 2019JOURNAL FREE ACCESSA syndiotactic-rich polystyrene (sPS) in a mixed solvent of toluene and chloroform converted to a thermoreversible gel from its semi-dilute solution. According to measurements of gel-melting temperatures, toluene behaved as a relatively poor solvent for sPS, while chloroform was a relatively good solvent. The critical gelation concentration C*, i.e., the minimum concentration for gel formation became higher with increasing the volume fraction of chloroform Xchloro. In order to characterize the sPS physical gel formed in a mixed solvent, the junction length ζ and ρ sequence were estimated as a function of the volume fraction Xchloro using the thermodynamic theory derived by Tanaka and Stockmayer. The theory suggested that the junction size S (ζ × ρ) was ca. 320–450 for sPS/toluene gel, 150–200 for sPS/chloroform gel, and the junction size S decreased with increasing Xchloro. In other words, the junction size S became larger when a gel was formed in a poor solvent. FT-IR spectral data suggest that sPS chain adopted the T2G2 conformation in the mixed solvents of toluene and chloroform, and then gelation occurred. FE-SEM images show a fibrillar morphology of the sPS gel.
 View full abstractDownload PDF (2892K)
View full abstractDownload PDF (2892K) -
Michihiro IIJIMA, Maiko KAWADA, Yuna SATO, MinLey PUA, Masayuki KAMEYA ...2019 Volume 76 Issue 4 Pages 276-287
Published: July 25, 2019
Released on J-STAGE: July 25, 2019
Advance online publication: June 04, 2019JOURNAL FREE ACCESSA method for the precision synthesis of hetero-telechelic poly(ethylene glycol) (PEG)s with a carboxyl group at the alpha-terminus was studied. Hydroxypivalic acid (HPA) that contains both a hydroxyl group and a carboxyl group, was used as the starting compound. Anionic ring opening polymerization of ethylene oxide (EO) proceeded quantitatively by dropping a solution of HPA into potassium naphthalate THF solution, which induces highly dispersible initiators. In addition, it is indicated that macromonomers with a polymerizable group at the omega-terminus of PEG were synthesized quantitatively. And furthermore, a synthetic method to produce branched hetero PEGs with one carboxyl group at the alpha-terminus and two PEG chains were established by using a similar method. These quantitative synthetic methods of hetero PEGs possessing a carboxyl group at the alpha-terminus are promising for the creation of highly functionalized materials in the future.
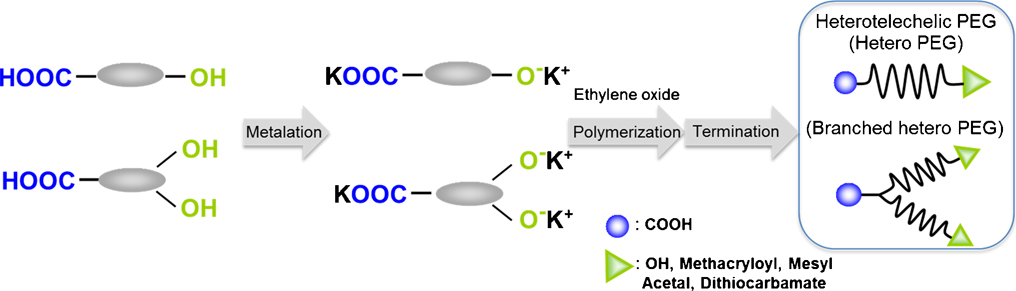 View full abstractDownload PDF (2936K)
View full abstractDownload PDF (2936K) -
Yoshihisa TAKEYAMA, Masahiro UENO, Mitsugu UEJIMA, Hirotada FUJIWARA, ...2019 Volume 76 Issue 4 Pages 288-296
Published: July 25, 2019
Released on J-STAGE: July 25, 2019
Advance online publication: June 10, 2019JOURNAL FREE ACCESSCarbon nanotubes (CNT) are a fibrous carbon material with very high aspect ratio due to having nano-size diameter and lengths of more than 1 µm. Therefore, CNT reinforced rubber shows high electrical conductivity, thermal conductivity and strength, even though the added CNT is a small fraction only. In this research, characteristics of CNT/rubber composites exposed to high-pressure hydrogen gas are assessed. As a result, it is confirmed that both, the equilibrium hydrogen content and the volume expansion, when exposed to high-pressure hydrogen gas, are excellent after uniformly dispersing SWCNT in the rubber, better than for other CNTs. Because of these pressure hydrogen characteristics, the CNT/rubber composites in this research are expected to be useful as highly durable seal materials in a high-pressure hydrogen environment.
 View full abstractDownload PDF (3664K)
View full abstractDownload PDF (3664K) -
Motomu TANAKA, Sara SENDA, Takatsugu ENDO, Takayuki TSUKEGI, Kazuaki N ...2019 Volume 76 Issue 4 Pages 297-304
Published: July 25, 2019
Released on J-STAGE: July 25, 2019
Advance online publication: June 25, 2019JOURNAL FREE ACCESSAs a completely biomass-based wood plastic composite material, a cellulose derivative (cellulose propionate, CP) composite material with added Erianthus fibers (EF) was developed. The biomass fiber EF was added to the cellulose derivative, and the rigidity of the cellulose derivative was greatly improved (tensile modulus improved by 149%). Although the strength decreased as the fiber content increased, it was revealed that it was possible to improve the tensile strength by preparing a cellulose derivative having an increased amount of residual hydroxyl groups and using it as a matrix component of a composite material (tensile strength is improved by 131%). Furthermore, it was confirmed that the thermal characteristics of this composite material are retained. Therefore, CP/Erianthus fiber achieved balanced thermal properties and better mechanical properties than petroleum-derived PP/MAPP composites.
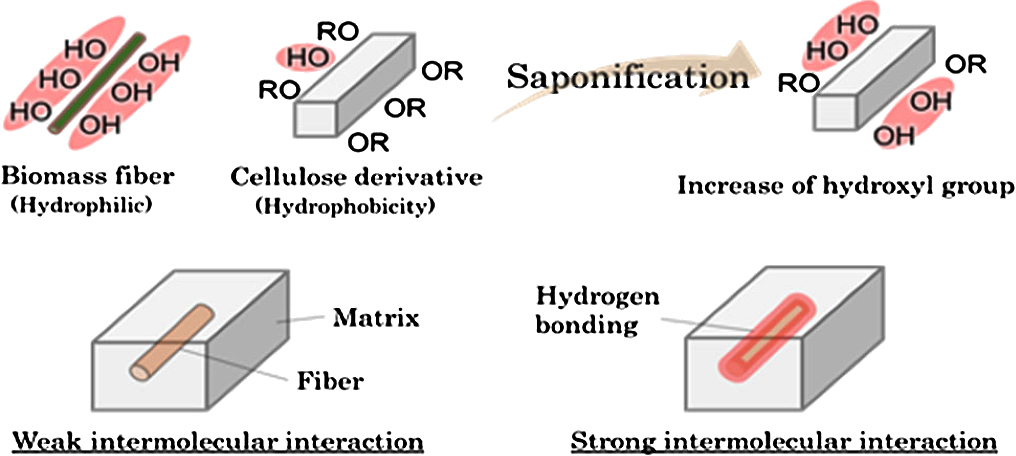 View full abstractDownload PDF (3120K)
View full abstractDownload PDF (3120K) -
Hiroki NAKAJIMA, Atsushi KAJIWARA2019 Volume 76 Issue 4 Pages 305-311
Published: July 25, 2019
Released on J-STAGE: July 25, 2019
Advance online publication: June 17, 2019JOURNAL FREE ACCESSElectron Spin Resonance (ESR) spectroscopic studies directed at clarifying the fundamentals of radical polymerizations have been conducted. Optimization of measurement conditions allows direct detection of radicals in polymerization reactions and well-resolved ESR spectra can be obtained. The spectra provide information not only on the structure, properties, and concentration of radicals but also information on the initiating and propagating (oligomeric and polymeric) radicals in radical polymerizations. A combination of ESR and atom transfer radical polymerization (ATRP) provided significant new information on the properties of radicals in radical polymerization, e.g. dependency of chain length, dynamics, and reactivity (hydrogen transfer) of propagating radicals. Up to now, it has been extremely difficult, even impossible, to obtain such information from ESR spectra during conventional radical polymerization. To overcome this difficulty radical precursors of oligo- and poly(meth)acrylates were prepared by ATRP and purified. Model radicals, with given chain lengths were generated by the reaction of well defined radical precursors with an organotin compound and were observed by ESR spectroscopy. tert-Butyl methacrylate (tBMA) radicals with various chain lengths showed clear chain length dependent ESR spectra. Especially, the ESR spectra of the dimeric model propagating radical were examined in detail at various temperatures.
 View full abstractDownload PDF (1901K)
View full abstractDownload PDF (1901K) -
Satoe ARATA, Atsushi KAJIWARA2019 Volume 76 Issue 4 Pages 312-318
Published: July 25, 2019
Released on J-STAGE: July 25, 2019
Advance online publication: May 16, 2019JOURNAL FREE ACCESSESR spectroscopic studies directed at clarifying the fundamentals of radical polymerizations have been conducted. Optimization of measurement conditions allows direct detection of radicals in polymerization reactions and well-resolved ESR spectra can be obtained. The spectra provide information not only on the structure, properties, and concentration of radicals but also information on the initiating and propagating (oligomeric and polymeric) radicals during radical polymerization. A combination of ESR and atom transfer radical polymerization (ATRP) provided significant new information on the properties of radicals in radical polymerizations, e.g. dependency of chain length, dynamics, and reactivity (hydrogen transfer) of propagating radicals. For acrylates, each ESR spectrum of dimeric, trimeric, tetrameric, and pentameric tert-butyl acrylate (tBA) model radicals observed at various temperatures provided clear experimental evidence of the 1,5-hydrogen shift. In this research work, ESR spectra observed during radical polymerization of tBA above 30°C, could be completely explained as being due to mid-chain radicals, using several experiments and a special simulation program.
 View full abstractDownload PDF (1412K)
View full abstractDownload PDF (1412K) -
Naofumi NAGA, Shun FUJIOKA, Megumi KOBAYASHI, Kazumasa MORIYAMA, Hidem ...2019 Volume 76 Issue 4 Pages 319-329
Published: July 25, 2019
Released on J-STAGE: July 25, 2019
Advance online publication: June 27, 2019JOURNAL FREE ACCESSInterpenetrating polymer network (IPN) gels have been synthesized by combination of networks formed by thiol-ene reaction of multifunctional compounds with bifunctional compounds (1st network) and ring opening polymerization of poly(ethylene glycol) diglycidylether (PEGDE) (2nd network). The 1st network was formed by thiol-ene click reaction of a multi-functional vinyl siloxane, (1,3,5,7-tetramethylcyclotetrasiloxane, TVMCTS) and an alkyl dithiol, (1,10-decane dithiol, DDT) in toluene. The mechanical properties of the IPN gels were investigated by compression tests. The IPN gels showed a higher breaking stress and breaking point than the single network gel. The 1st network has been also synthesized by the thiol-ene reaction of multi-functional thiol compounds and poly(ethylene glycol) diacrylate in some organic solvents (toluene, tetrahydrofuran). These IPN gels also showed higher breaking stress and breaking point in comparison with the corresponding single network gels. The IPN gels with PTMB showed higher Young’s modulus due to the physical entanglement between the 1st and 2nd networks. The reaction system of caster oil-hexamethylene diisocyanate (HDI)/epoxized soybean oil (ESO) was also usable to synthesize IPN gels. The 1st and 2nd networks were formed by the addition reaction of caster oil and HDI, and ring opening polymerization of ESO, respectively. The composition ratio and formation order of the 1st and 2nd network affected the mechanical properties of the resulting IPN gels.
 View full abstractDownload PDF (3208K)
View full abstractDownload PDF (3208K) -
Masahiro YOSHIZAWA-FUJITA, Hideki HANABUSA, Yuko TAKEOKA, Masahiro RIK ...2019 Volume 76 Issue 4 Pages 330-334
Published: July 25, 2019
Released on J-STAGE: July 25, 2019
Advance online publication: May 24, 2019JOURNAL FREE ACCESSFive kinds of protic ionic liquids (PILs) were synthesized by the neutralization reaction of amidine and carboxylic acid (acetic acid, propionic acid, or butyric acid), and the esterification reaction of cellulose, using acetic anhydride, was carried out in PILs. The obtained cellulose derivatives were soluble in DMSO. In the 1H NMR spectra of the cellulose derivatives, not only the chemical shift of acetyl group derived from acetic anhydride, but also the chemical shift, based on the anion of PILs, were observed. The degree of substitution (DS) of the produced cellulose derivatives varied depending on the structure of PILs. The obtained cellulose derivatives showed a thermal decomposition temperature above 270°C and a glass transition temperature below 170°C.
 View full abstractDownload PDF (860K)
View full abstractDownload PDF (860K) -
Effects of Addition of Large Particles on Lamellar Microphase-Separated Structure of Block CopolymerKenta TAMADA, Naoya TORIKAI, Masami KAWAGUCHI2019 Volume 76 Issue 4 Pages 335-340
Published: July 25, 2019
Released on J-STAGE: July 25, 2019
Advance online publication: June 04, 2019JOURNAL FREE ACCESSThe influence of added divinylbenzene (DVB) cross-linked particles, having a diameter of 3 µm and 30 µm, on the lamellar microphase-separated structure of styrene-1,4-rich-isoprene (SI) diblock copolymer is investigated. The DVB particles readily sedimented in toluene solution of SI due to low dispersibility, therefore the particles were localized in the lower part of the solvent-cast films. The lamellar structure, formed in the process, exhibited preferential orientation along the direction parallel to the film surface, and the domain spacing along the perpendicular direction was lower than in the parallel direction. The degree of preferential lamellar orientation was lowered by adding the DVB particles, leading to an increment in the domain spacing. Furthermore, the DVB particles with larger diameter gave lower domain spacing, though they produced a lower degree of lamellar orientation.
 View full abstractDownload PDF (1766K)
View full abstractDownload PDF (1766K) -
Kyoung-Mo JEONG, Sun-Goo KIM, Jeong Yong RYU, Yong Kyu LEE, Ken’ichi K ...2019 Volume 76 Issue 4 Pages 341-348
Published: July 25, 2019
Released on J-STAGE: July 25, 2019
Advance online publication: July 03, 2019JOURNAL FREE ACCESSThe aim of this study is to evaluate the adhesive properties of UV-curing liquid (ink) on a non-porous surface. We examined how the adhesive strength was affected by the ink characteristics, as different type mono-functional monomers were used in the ink composition. The flexibility of the cured film structure seems to be crucial for improving adhesive strength, because a flexible structure can relax the internal strain that exist between the substrate and the cured film surface. It seem that when we use methacrylate type mono-functional monomers (CHMA, HEMA) in the ink composition, the two monomers play an important role in the ink system in order to reduce the crosslinking density of the cured film. Also, we understood that acrylate type mono-functional monomers (CHA, HBA) were effective to increase adhesive strength because of their low surface tension and less shrinkage, although the two inks containing each monomers formed relatively higher crosslinking density of cured films than the methacrylate type ones.
 View full abstractDownload PDF (1217K)
View full abstractDownload PDF (1217K)
-
Ikue MOTOMURA, Kimie SHIOMI, Shuichi MAEDA, Koji ABE2019 Volume 76 Issue 4 Pages 349-355
Published: July 25, 2019
Released on J-STAGE: July 25, 2019
Advance online publication: May 14, 2019JOURNAL FREE ACCESSWe synthesized polyoxamides (PX) using various diamines and diphenyl-oxalate (DPO) and investigated their molecular weight, copolymer composition, thermal properties, mechanical properties, swelling behavior for electrolytes of lithium ion batteries (LIB) and adhesive properties with electrodes of LIB. We established a new method to synthesize PX with high molecular weight by using N-methylpyrrolidone as a synthesis solvent, setting temperature to 200 ° C and devising the order of charging diamine and DPO. All PXs synthesized in this study were crystalline polymers, harder than polyvinylidene-di-fluoride (PVdF) and had smaller elongation than PVdF. Both, the saturated swelling ratios of PX for electrolytes of LIB and the changes in mechanical properties due to swelling were small. We found in this study that the adhesive properties of PX copolymers composed of 1,3-bisaminomethylcyclohexane and hexamethylenediamine on both cathode and anode were good, but they were inferior to that of PVdF.
 View full abstractDownload PDF (498K)
View full abstractDownload PDF (498K) -
Youta IWAMOTO, Takashi UBUKATA2019 Volume 76 Issue 4 Pages 356-361
Published: July 25, 2019
Released on J-STAGE: July 25, 2019
Advance online publication: June 13, 2019JOURNAL FREE ACCESSSurface reliefs (SR) in crystalline diacetylene (DA) films have been investigated. Blue polydiacetylene (PDA) was locally formed by irradiation of ultraviolet light through a photomask to DA crystalline films. Exposing these films to heat or organic solvent vapor led to surface relief formation by transfer of the DA in the unexposed area to the exposed area accompanied by the PDA color transition from blue to red. A highly photosensitive SR formation was attained and stable SR in the crystalline DA films were obtained.
 View full abstractDownload PDF (2189K)
View full abstractDownload PDF (2189K)
- |<
- <
- 1
- >
- >|