
- |<
- <
- 1
- >
- >|
-
2021 年 107 巻 1 号 p. Cover-
発行日: 2021/01/01
公開日: 2020/12/31
ジャーナル オープンアクセスPDF形式でダウンロード (378K) -
2021 年 107 巻 1 号 p. Contents-
発行日: 2021/01/01
公開日: 2020/12/31
ジャーナル オープンアクセスPDF形式でダウンロード (3047K) -
2021 年 107 巻 1 号 p. Editorial-
発行日: 2021/01/01
公開日: 2020/12/31
ジャーナル オープンアクセスPDF形式でダウンロード (232K)
-
Wang Zhanjun, Sohn Il2021 年 107 巻 1 号 p. 1-14
発行日: 2021年
公開日: 2020/12/31
[早期公開] 公開日: 2020/09/10ジャーナル オープンアクセス HTMLSeveral recently developed highly alloyed steel grades have shown unsurpassed performance in terms of physical, chemical, and electromagnetic properties. However, broader commercialization of these steels has been hampered by limitations in mold flux performance. Newly developed steels containing considerable amounts of dissolved Al, Mn, and Ti actively react with typical CaO-SiO2-based mold fluxes, which severely changes the composition and subsequently the thermophysical properties of the mold flux that determine the external and internal quality of the as-cast steels. These dynamic changes result in nonuniform heat transfer, lubrication issues, surface defects, and caster breakouts. This work critically assesses the current status of the high-temperature thermophysical properties of CaO-SiO2-based and CaO-Al2O3-based mold fluxes intended for use in casting highly alloyed steel grades. Thermophysical properties, including viscosity, crystallization, thermal conductivity, and heat flux, have been evaluated. The effect of compositional variables including CaO/SiO2, CaO/Al2O3, and Al2O3/SiO2 mass ratios and the additions of CaF2, B2O3, Li2O, K2O, Na2O, TiO2, and BaO on these high-temperature thermophysical properties are discussed.
 抄録全体を表示PDF形式でダウンロード (1995K) HTML形式で全画面表示
抄録全体を表示PDF形式でダウンロード (1995K) HTML形式で全画面表示
-
坪内 直人, 永沼 遼, 望月 友貴, 林崎 秀幸, 宍戸 貴洋, Atul Sharma2021 年 107 巻 1 号 p. 15-23
発行日: 2021年
公開日: 2020/12/31
[早期公開] 公開日: 2020/10/28ジャーナル オープンアクセス HTMLIn order to produce high-strength coke from low-quality coals, noncovalent bonds between O-functional groups in coal were cleaved by pyridine containing HPC pyridine soluble and HPC-derived thermoplastic components were introduced into the pores formed by swelling; thus, the synergistic effect during carbonization of the suppression of cross-linking reactions and the fluidity amplification due to close placement of coal and thermoplastic components was investigated. When HPC was extracted with pyridine, a decrease in O-functional groups was observed in the pyridine-soluble and pyridine-insoluble components. When HPC was extracted with MeOH, on the other hand, O-functional groups in HPC selectively moved into the soluble components. When non- or slightly-caking coal was chemically-modified with the prepared HPC pyridine-soluble components by utilizing the solvent-swelling effect of pyridine, the fluidity improved compared with the coals physically mixed with the soluble components or HPC. On the other hand, the fluidity of the chemically-modified sample with the MeOH-soluble components hardly changed from that of the original sample, and no effect of the modification with the thermoplastic component was observed. Furthermore, it was clarified that higher-strength coke can be produced from the chemically-modified sample with the HPC pyridine-soluble components than from the original coal or the physically mixed coal with the soluble components. The contraction behavior during carbonization of the chemically-modified sample with the soluble components and that of the original coal was investigated; as a result, a large difference was not observed between these two. Thus, it was found that high-strength coke can be produced from low-quality coals by the present method.
 抄録全体を表示PDF形式でダウンロード (3444K) HTML形式で全画面表示
抄録全体を表示PDF形式でダウンロード (3444K) HTML形式で全画面表示 -
坪内 直人, 永沼 遼, 望月 友貴, 林崎 秀幸, 宍戸 貴洋2021 年 107 巻 1 号 p. 24-34
発行日: 2021年
公開日: 2020/12/31
[早期公開] 公開日: 2020/10/28ジャーナル オープンアクセス HTMLIn this work, we studies the production of higher-strength coke from chemically-loaded coal in which noncovalent-bonds between O-functional groups in coal are cleaved by pyridine and HPC-derived thermoplastic components are introduced into the pores produced by swelling. The effect of heating rate up to thermoplasticity temperatures of coal on coke strength is first investigated. To examine synergistic effects due to further fluidity enhancements caused by the increased proximity of coal to thermoplastic components during carbonization, the influence of heating rate on coke-strength prepared from pelleted-coal also examined, as described above, to clarify the optimal heating conditions for yielding high-strength coke from slightly-caking coal. An investigation of the use of a SUS-tube to produce high-strength coke from slightly-caking coal with chemically-loaded HPC pyridine-soluble components reveals that high-strength coke may be obtained by 20ºC/min to 400ºC and then continuing to heat at 3ºC/min to 1000ºC. On the other hand, when producing coke from formed specimens consisting of slightly-caking coal with chemically-loaded HPC pyridine-soluble components, we exhibit that, by heating first at 20ºC/min to 500-600ºC and then heating at 3ºC/min to 900ºC, it is possible to produce coke whose strength rivals that of coke produced by carbonization at 3ºC/min of strongly-caking coal. In addition, in producing high-strength coke from formed slightly-caking coal, an optimal amount of additive is present for all types of additive considered – HPC physical blend, chemically-loaded pyridine-soluble HPC and physical blend of pyridine-insoluble HPC components – and, with chemically-loaded pyridine-soluble HPC, it is possible to prepare particularly high-strength coke.
 抄録全体を表示PDF形式でダウンロード (3538K) HTML形式で全画面表示
抄録全体を表示PDF形式でダウンロード (3538K) HTML形式で全画面表示 -
坪内 直人, 永沼 遼, 望月 友貴, 林崎 秀幸, 宍戸 貴洋2021 年 107 巻 1 号 p. 35-43
発行日: 2021年
公開日: 2020/12/31
[早期公開] 公開日: 2020/10/28ジャーナル オープンアクセス HTMLIn the present study, we prepare several types of specimens from non-caking coal – including specimens in which noncovalent bonds between O-functional groups in coal are cleaved by pyridine and HPC-derived thermoplastic components are introduced into the pores produced by swelling, as well as specimens consisting of physical blends with HPC – and examine the influence of heating conditions and types of caking agents on the production of high-strength coke using a SUS tube. We also investigate the influence of heating conditions and types of caking agents on the strength of coke from pelleted specimens and determine the optimal conditions for producing high-strength coke from non-caking coal. HPC with a wide range of thermoplastic properties is more effective as caking agents than additives containing only low-molecular-weight or high-molecular-weight components. In addition, the strength of the produced coke depends on the amount of the additive, and optimal values of the additive amount are present. It was found that the following heating schedule is effective for producing high-strength coke from non-caking coal with added caking agents: First, high-speed heating (20ºC/min) to an intermediate temperature in the range 400-600ºC, recognized as the thermoplastic temperature range for typical caking coal; then, low-speed heating (3ºC/min) to the temperature range of 900-1000ºC. Moreover, we demonstrate that, by increasing the rate of heating in the thermoplastic temperature range, it is possible to reduce the amount of caking agent added.
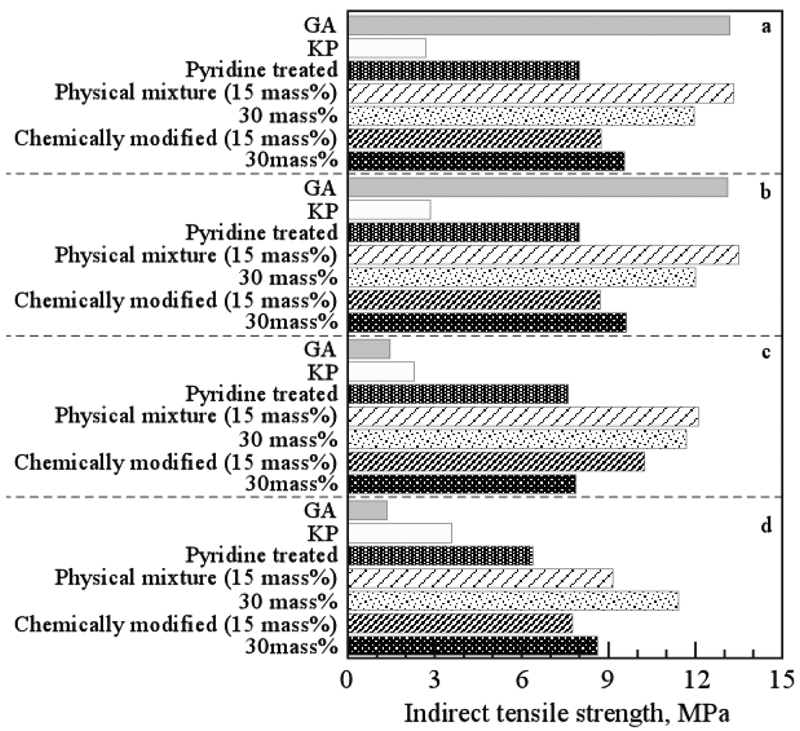 抄録全体を表示PDF形式でダウンロード (2989K) HTML形式で全画面表示
抄録全体を表示PDF形式でダウンロード (2989K) HTML形式で全画面表示 -
土肥 勇介, 深田 喜代志, 松井 貴, 角 広行, 下山 泉2021 年 107 巻 1 号 p. 44-52
発行日: 2021年
公開日: 2020/12/31
ジャーナル オープンアクセス HTMLIn our previous study, it was revealed that high MF coal having longer “maximum permeation distance”, which was developed as a unique thermoplasticity index, forms lower roundness pores and thinner pore-wall structures in coke and that coke strength deteriorated when the coal blend included the longer maximum permeation distance coal. Therefore, techniques for reducing the adverse effects of long maximum permeation distance coal on coke strength are essential so as to utilize the coal more efficiently. Some practical techniques of design and control regarding coal grain size were developed for ameliorating coking property of long maximum permeation distance coal in our previous paper. The techniques are based on the facts that the coke strength deterioration caused by long maximum permeation distance coal in coal blend was suppressed with decreasing the coal size.
In this paper, influence of weathering, which is mild oxidation with air atmosphere, on permeation distance and coke strength were researched in order to clarify possibilities of controlling maximum permeation distance in another way of the techniques of coal size adjustment. As a result, it was found that the measured maximum permeation distance and coke strength deterioration caused by long permeation distance coal was reduced by weathering processing although the fluidity was impaired. Accordingly, some control techniques of maximum permeation distance by weathering were proposed for more effective utilization of long maximum permeation distance coal.
 抄録全体を表示PDF形式でダウンロード (4626K) HTML形式で全画面表示
抄録全体を表示PDF形式でダウンロード (4626K) HTML形式で全画面表示
-
水上 英夫, 白井 善久, 平城 正2021 年 107 巻 1 号 p. 53-63
発行日: 2021年
公開日: 2020/12/31
ジャーナル オープンアクセス HTMLThe occurrence of longitudinal surface cracks in hypo-peritectic carbon steel slabs depends largely on the cooling capacity of the mold and the flow velocity of molten steel below the meniscus. The influence of both flow velocity of the molten steel below the meniscus and the heat flux in the copper mold were examined using continuous casting tests and numerical simulation of the molten steel flow. The casting speed was fixed, and the meniscus flow velocity was controlled by adjusting the port size of the submerged entry nozzle. The molten steel flow velocity was predicted by a three-dimensional unsteady-state numerical simulation. Heat flux in the copper mold was calculated based on temperature readings from thermocouples arranged in the direction of both the mold width and mold length. When the difference in flow velocity of molten steel in the mold width direction became large, longitudinal surface cracks occurred in the central region of the slab. In these cases, the heat flux below the meniscus in the mold width direction was not constant. Small holes were drilled along the central region of the mold width. This decreased both the heat flux and tensile strength of the central region of the slab width, and successfully reduced the occurrence of longitudinal surface cracks.
 抄録全体を表示PDF形式でダウンロード (2032K) HTML形式で全画面表示
抄録全体を表示PDF形式でダウンロード (2032K) HTML形式で全画面表示 -
大塲 康英, 吉岡 孝宜, 松井 隆助, 濱屋 大輔2021 年 107 巻 1 号 p. 64-72
発行日: 2021年
公開日: 2020/12/31
[早期公開] 公開日: 2020/10/02ジャーナル オープンアクセス HTMLContinuous casting of high-carbon steel containing 1% carbon tends to be operated with low mold flux consumption, resulting in insufficient lubricity. In this study, a mold flux was developed by increasing the viscosity to form a glassy film easily and improve the lubricity between the mold and the solidified shell. In the casting with the developed mold flux, a film with a thickness of 3 mm was stably formed inside the mold to cover the meniscus, bleeding was prevented, and the frequency of surface crack defects on the bloom was reduced by 80%. In the film of the developed mold flux, a 1.2 mm liquid layer lubricated the initial solidification shell.The increased thermal resistance at the film-mold interface reduced the heat flux in the mold, which contributed to the uniform initial solidification. The formation and the growth mechanisms of the crystalline layer in the film were as follows. Firstly, the mold flux flows into the gap between the mold and the initial solidification shell and forms a glassy film. Subsequently, crystallization of the glassy film starts from the mold plate side. Thereafter, the crystallization progresses in the thickness direction of the film until the position of the solidification temperature of the mold flux. The existence of two phases, a liquid layer and a solid layer, played an important role to achieve high lubricity and uniform initial solidification.
 抄録全体を表示PDF形式でダウンロード (5046K) HTML形式で全画面表示
抄録全体を表示PDF形式でダウンロード (5046K) HTML形式で全画面表示
-
山田 克美, 井原 直哉2021 年 107 巻 1 号 p. 73-81
発行日: 2021年
公開日: 2020/12/31
ジャーナル オープンアクセス HTMLFE-EPMA combined with Soft X-ray Emission Spectrometry (SXES) has been applied to quantification of nitrogen for soft gas nitriding steels and its penetration behavior was discussed quantitatively over 0.03 mass% region. Change in intensity of N-Kα(n=2) peak within several hundred micrometer depth of the conventional soft gas nitriding steel was consistent with not only hardness change but also quantification result of N as nitrides determined by chemical analysis on extracted residue. The contents of nitrogen at surface region were determined of the steels with different nitriding conditions by using a calibration curve and those depth profiles of nitrogen showed the same tendency in hardness and microstructure evolution. It was concluded that SXES was quite useful for bulk nitrogen analysis of gas nitriding steels at micrometer resolution.
 抄録全体を表示PDF形式でダウンロード (3691K) HTML形式で全画面表示
抄録全体を表示PDF形式でダウンロード (3691K) HTML形式で全画面表示 -
板橋 大輔, 村尾 玲子, 谷口 俊介, 水上 和実, 高木 秀彰, 木村 正雄2021 年 107 巻 1 号 p. 82-91
発行日: 2021年
公開日: 2020/12/31
[早期公開] 公開日: 2020/10/30ジャーナル オープンアクセス HTMLTo investigate the performance of the size measurement by asymmetric flow field-flow fractionation (AF4), the measurement results of gold nanoparticles were compared among AF4, transmission electron microscopy (TEM), and small-angle X-ray scattering (SAXS) in terms of the average size and full width at half maximum (FWHM) of the size distribution. Although the average size was almost the same for the three methods, the FWHM measured using AF4 was larger than those measured using TEM and SAXS. This is attributed to the diffusion of the gold nanoparticles inside the AF4 instruments. The broadening factor of AF4 analysis was determined as 2.08 by the average of FWHM ratio of AF4 to TEM measured using the several sphere-like gold nanoparticles. In addition, the effect of particle shape on the above broadening factor was investigated using the sphere-like and plate-like silver nanoparticles. The broadening factor for plate-like particles apparently became smaller than that for sphere-like particles, possibly because the Brownian motion of plate-like particles was suppressed.
Furthermore, the AF4 analysis with the FWHM correction method using the broadening factor was applied to niobium carbide (NbC) precipitates in steels. The average size measured by AF4 was mostly consistent with the results obtained in regions observed by TEM. Moreover, an increase in the number density of nanometer-sized NbC by heat treatment was successfully detected. The effect of particle shape on FWHM should be further investigated and improved; however, AF4 with the broadening factor can semi-quantitatively analyze the size distribution of nanoprecipitates in steels.
 抄録全体を表示PDF形式でダウンロード (2896K) HTML形式で全画面表示
抄録全体を表示PDF形式でダウンロード (2896K) HTML形式で全画面表示
-
川﨑 大輝, 松浦 宏行2021 年 107 巻 1 号 p. 92-102
発行日: 2021年
公開日: 2020/12/31
[早期公開] 公開日: 2020/10/29ジャーナル オープンアクセス HTMLTo recover the barren coast or degraded paddy field, the supply of nutrient elements such as Fe is effective. Since steelmaking slag contains various kinds of potent elements, it is expected to be used as an environmental restoration material. The dissolution mechanism of various elements from slag and the influence of surrounding organic matters and microorganisms must be clarified to utilize steelmaking slag in these methods effectively. In this research, the dissolution tests were conducted by using synthesized CaO-SiO2-FeO-Al2O3-P2O5 amorphous slag samples and aqueous solutions containing gluconic acid. Concentration of various elements in the solution, especially Fe, increased by the addition of gluconic acid. To evaluate the effect of gluconic acid on the dissolution behavior quantitatively, the existence forms of various elements in the solution were thermodynamically estimated based on the experimental results. The maximum ratio of chelated iron to total iron was 97%, indicating that the increase in iron concentration by adding gluconic acid was owing to the formation of iron complex ions. On the contrary, concentrations of Ca, showing complex formation ratio low, or Si and P, for which the complex formation has not been reported, also increased by adding the acid. This suggested the existence of elution mechanisms other than complex formation.
 抄録全体を表示PDF形式でダウンロード (4698K) HTML形式で全画面表示
抄録全体を表示PDF形式でダウンロード (4698K) HTML形式で全画面表示
- |<
- <
- 1
- >
- >|