
- |<
- <
- 1
- >
- >|
-
2022 年 108 巻 4 号 p. Cover-
発行日: 2022/04/01
公開日: 2022/03/31
ジャーナル オープンアクセスPDF形式でダウンロード (663K) -
2022 年 108 巻 4 号 p. Contents-
発行日: 2022/04/01
公開日: 2022/03/31
ジャーナル オープンアクセスPDF形式でダウンロード (2017K) -
2022 年 108 巻 4 号 p. Editorial-
発行日: 2022/04/01
公開日: 2022/03/31
ジャーナル オープンアクセスPDF形式でダウンロード (204K)
-
桑原 利彦2022 年 108 巻 4 号 p. 233-248
発行日: 2022年
公開日: 2022/03/31
ジャーナル オープンアクセス HTMLForming simulation is an indispensable analysis tool in industry. The most important thing in forming simulation is to reproduce the deformation behavior of the material during forming as accurately as possible. This paper reviews various material test methods developed from the viewpoint of improving the accuracy of material models for sheet metals, and the deformation characteristics and material modeling of steel sheets. Section 2 describes the difference between the yield surface and the contour of plastic work to explain the advantages of constructing a material model based on the latter. Section 3 gives an overview of various material test methods for measuring the contour of plastic work of sheet metals. Sections 4 and 5 show examples of measuring the deformation behavior of steel sheets in linear and nonlinear stress paths, and related literature.
 抄録全体を表示PDF形式でダウンロード (8683K) HTML形式で全画面表示
抄録全体を表示PDF形式でダウンロード (8683K) HTML形式で全画面表示
-
朴 賢祐, 金 勁賢, 朴 亨原, 丁 晟, 柳本 潤2022 年 108 巻 4 号 p. 249-259
発行日: 2022年
公開日: 2022/03/31
[早期公開] 公開日: 2022/01/28ジャーナル オープンアクセス HTMLA precise flow curve for a wide range of forming conditions is important for accurately predicting forming force. Moreover, since the flow curve reflects microstructural changes, its accurate description must be obtained under various temperatures and strain rates up to 300 s−1. For practical forming processes such as hot strip rolling and wire rod rolling, the deformation behavior at high strain rates (50–200 s−1) must also be studied. However, a uniform axial high strain rate is difficult to achieve. Hence, a new deceleration method is developed. Also, the compression test at high strain rates is accompanied by marked internal heat generation, therefore, temperature and deformation are highly inhomogeneous compared with those in tests at lower strain rates. In addition to this problem, heat conduction to the die and friction should be corrected using inverse analysis. By considering the internal temperature increase effect at high strain rates, the uniaxial flow curve obtained using inverse analysis is shown to be greater than the experimental apparent stress–axial strain curve. And then, a regression method is applied to obtain a generalized flow curve at high strain rates, which can cover wider ranges of strain rates and temperatures. Finally, they are compared with an extrapolated flow curve that is regressed using an intermediate strain rate in our previous research. By comparing those results, the extrapolated flow curve is greatly different from the flow curve obtained in the current research. To find the reason for the difference, a microstructure analysis using EBSD is implemented.
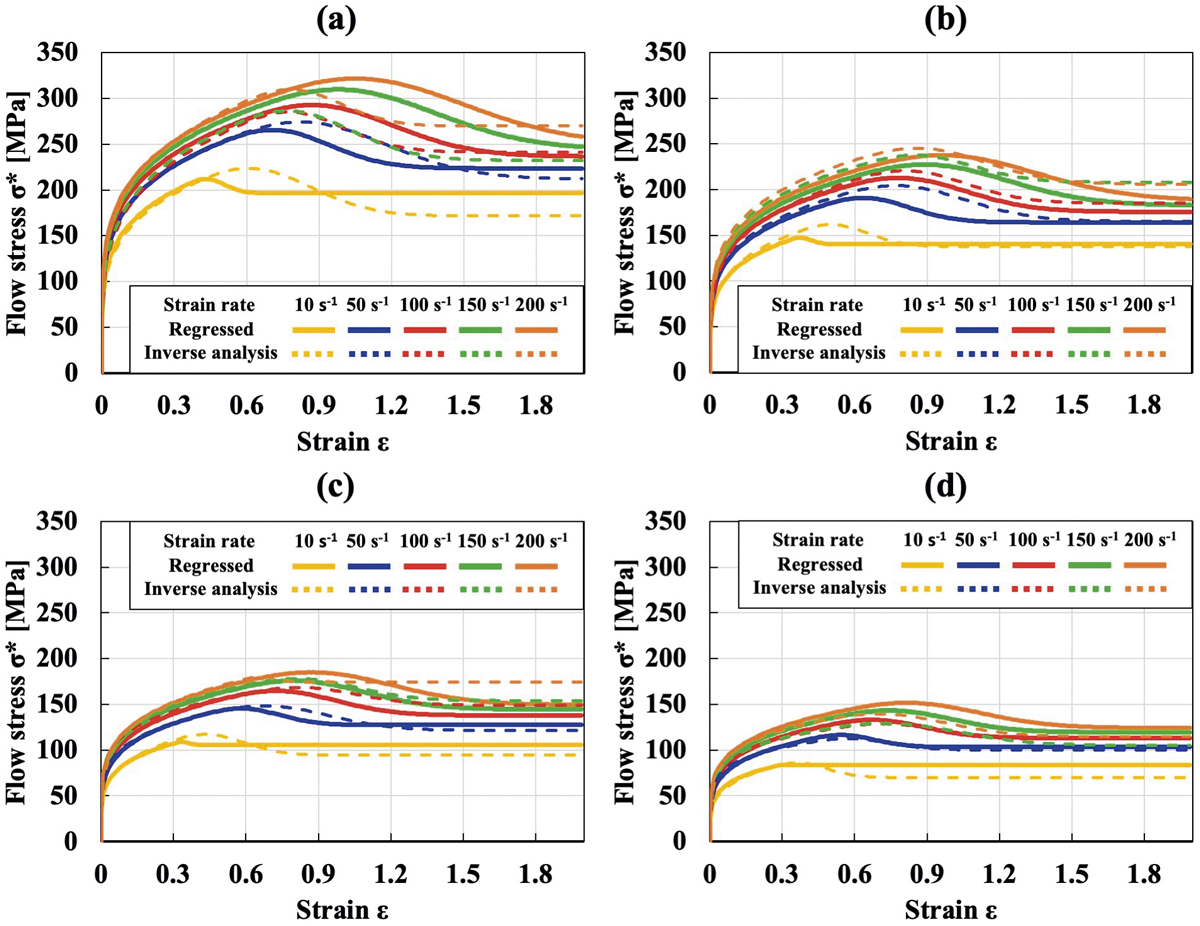 抄録全体を表示PDF形式でダウンロード (10917K) HTML形式で全画面表示
抄録全体を表示PDF形式でダウンロード (10917K) HTML形式で全画面表示
-
片山 英樹, 勝村 俊規, 明石 孝也, 堤 祐介2022 年 108 巻 4 号 p. 260-267
発行日: 2022年
公開日: 2022/03/31
ジャーナル オープンアクセス HTMLThe surface potential measurement has been applied for elucidation the hydrogen entry behavior of pure iron under wet-dry cyclic corrosive environment. The hydrogen detection side was electroplated with Ni to prevent any changes of the surface due to a long-time experiment. Distilled water was used in the corrosion test after adding the droplet of NaCl solution in the first cycle. Although the first cycle gave the surface potential change immediately after the surface dried, after 2 cycles the change occurred while the surface was still wet. The potential change area corresponded to the area that showed metallic luster. The surface potential further decreased in the drying process, and the change area also was expanded. In the third cycle, the surface potential changed even on the back side of the surface covered with the corrosion product. It is possible to two-dimensionally detect and visualize the change in permeated hydrogen with time under atmospheric corrosive environment by the surface potential measurement.
 抄録全体を表示PDF形式でダウンロード (1006K) HTML形式で全画面表示
抄録全体を表示PDF形式でダウンロード (1006K) HTML形式で全画面表示 -
裵 聖和, 大上 悟, 谷ノ内 勇樹, 孫 仁俊, 中野 博昭2022 年 108 巻 4 号 p. 268-281
発行日: 2022年
公開日: 2022/03/31
ジャーナル オープンアクセス HTMLZn–Ni alloys were electrodeposited on a Cu electrode at 10–5000 A·m−2, 5 × 104 C·m−2, and 293, 313 and 333 K in unagitated zincate solutions containing the reaction product of epichlorohydrin and imidazole (IME) as a brightener. The synergistic effect of IEM and solution temperature on the deposition behavior of Zn–Ni alloy was investigated. The transition current density, at which the deposition behavior shifted from the normal type to anomalous ones, decreased with IME at 293 K, but didn’t change regardless of IME addition at 313 and 333 K. The suppression effect of IME on the Zn and Ni depositions during alloy deposition was observed at 293 K, while at 313 and 333 K, the suppression effect was decreased on the Zn deposition but was maintained on the Ni deposition. Therefore, Ni content in deposits significantly decreased with IME as temperature increased. The current efficiency for Zn deposition significantly decreased with IME at 293 K, while the degree of decrease was small at 313 and 333 K. The content of C in deposits was highest at 293 K, and decreased with increasing solution temperature, indicating that the adsorption ability of IME on the cathode decreases with increasing temperature. As a result, the suppression effect of IME on the Zn deposition seems to decrease with increasing temperature. The gloss of deposited films was highest at 293 K. This is attributed to adsorption ability of IME being large at 293 K and deposited films with fine crystals becoming smooth.
 抄録全体を表示PDF形式でダウンロード (5929K) HTML形式で全画面表示
抄録全体を表示PDF形式でダウンロード (5929K) HTML形式で全画面表示
-
上島 良之2022 年 108 巻 4 号 p. 282-285
発行日: 2022年
公開日: 2022/03/31
ジャーナル オープンアクセス HTMLA certain relation was found between melting temperature of multi-component oxides and their basicity index evaluated from the viewpoint of chemical bonding. It can be used as a convenient method to know the melting temperature of multi-component oxides for which thermodynamic information is lacking.
 抄録全体を表示PDF形式でダウンロード (729K) HTML形式で全画面表示
抄録全体を表示PDF形式でダウンロード (729K) HTML形式で全画面表示
- |<
- <
- 1
- >
- >|