
- Issue 16 Pages 1251-
- Issue 15 Pages 1165-
- Issue 14 Pages 1033-
- Issue 13 Pages 989-
- Issue 12 Pages 899-
- Issue 11 Pages 795-
- Issue 10 Pages 721-
- Issue 9 Pages 653-
- Issue 8 Pages 569-
- Issue 7 Pages 513-
- Issue 6 Pages 427-
- Issue 5 Pages 395-
- Issue 4 Pages 343-
- Issue 3 Pages 89-
- Issue 2 Pages 35-
- Issue 1 Pages 1-
- Issue 16 Pages 2157-
- Issue 15 Pages 1805-
- Issue 14 Pages 1613-
- Issue 13 Pages S1141-
- Issue 12 Pages S1037-
- Issue 11 Pages 1443-
- Issue 10 Pages 1273-
- Issue 9 Pages 1077-
- Issue 8 Pages 917-
- Issue 7 Pages 751-
- Issue 6 Pages 585-
- Issue 5 Pages S389-
- Issue 4 Pages S1-
- Issue 3 Pages 403-
- Issue 2 Pages 233-
- Issue 1 Pages 19-
- Issue 16 Pages 2153-
- Issue 15 Pages 1977-
- Issue 14 Pages 1813-
- Issue 13 Pages S1285-
- Issue 12 Pages S1043-
- Issue 11 Pages 1667-
- Issue 10 Pages 1481-
- Issue 9 Pages 1231-
- Issue 8 Pages 891-
- Issue 7 Pages 711-
- Issue 6 Pages 538-
- Issue 5 Pages S407-
- Issue 4 Pages S1-
- Issue 3 Pages 347-
- Issue 2 Pages 173-
- Issue 1 Pages 14-
- Issue 16 Pages 1837-
- Issue 15 Pages 1711-
- Issue 14 Pages 1569-
- Issue 13 Pages S1205-
- Issue 12 Pages S1034-
- Issue 11 Pages 1423-
- Issue 10 Pages 1269-
- Issue 9 Pages 1059-
- Issue 8 Pages 925-
- Issue 7 Pages 775-
- Issue 6 Pages 627-
- Issue 5 Pages S287-
- Issue 4 Pages S1-
- Issue 3 Pages 301-
- Issue 2 Pages 147-
- Issue 1 Pages 12-
- Issue 16 Pages 2179-
- Issue 15 Pages 1795-
- Issue 14 Pages 1631-
- Issue 13 Pages S1053-
- Issue 12 Pages S1023-
- Issue 11 Pages 1501-
- Issue 10 Pages 1315-
- Issue 9 Pages 987-
- Issue 8 Pages 767-
- Issue 7 Pages 621-
- Issue 6 Pages 473-
- Issue 5 Pages S305-
- Issue 4 Pages S1-
- Issue 3 Pages 299-
- Issue 2 Pages 151-
- Issue 1 Pages 16-
- Issue 16 Pages 1945-
- Issue 15 Pages 1699-
- Issue 14 Pages 1531-
- Issue 13 Pages S1055-
- Issue 12 Pages S1013-
- Issue 11 Pages 1367-
- Issue 10 Pages 1215-
- Issue 9 Pages 1087-
- Issue 8 Pages 887-
- Issue 7 Pages 721-
- Issue 6 Pages 507-
- Issue 5 Pages S317-
- Issue 4 Pages S1-
- Issue 3 Pages 343-
- Issue 2 Pages 187-
- Issue 1 Pages 17-
- Issue 16 Pages 2405-
- Issue 15 Pages 2067-
- Issue 14 Pages 1865-
- Issue 13 Pages 1675-
- Issue 12 Pages S1055-
- Issue 11 Pages S1015-
- Issue 10 Pages 1479-
- Issue 9 Pages 1129-
- Issue 8 Pages 895-
- Issue 7 Pages 711-
- Issue 6 Pages 545-
- Issue 5 Pages S325-
- Issue 4 Pages S1-
- Issue 3 Pages 369-
- Issue 2 Pages 193-
- Issue 1 Pages 16-
- Issue 16 Pages 2573-
- Issue 15 Pages 2261-
- Issue 14 Pages 2073-
- Issue 13 Pages S1111-
- Issue 12 Pages S1001-
- Issue 11 Pages 1867-
- Issue 10 Pages 1657-
- Issue 9 Pages 1409-
- Issue 8 Pages 1043-
- Issue 7 Pages 841-
- Issue 6 Pages 649-
- Issue 5 Pages S415-
- Issue 4 Pages S1-
- Issue 3 Pages 431-
- Issue 2 Pages 225-
- Issue 1 Pages 3-
- |<
- <
- 1
- >
- >|
-
2022Volume 108Issue 8 Pages Cover-
Published: August 01, 2022
Released on J-STAGE: July 31, 2022
JOURNAL OPEN ACCESSDownload PDF (663K) -
2022Volume 108Issue 8 Pages Contents-
Published: August 01, 2022
Released on J-STAGE: July 31, 2022
JOURNAL OPEN ACCESSDownload PDF (2712K) -
2022Volume 108Issue 8 Pages Editorial-
Published: August 01, 2022
Released on J-STAGE: July 31, 2022
JOURNAL OPEN ACCESSDownload PDF (207K)
-
Takahiro MikiArticle type: Preface
2022Volume 108Issue 8 Pages 439
Published: 2022
Released on J-STAGE: July 31, 2022
JOURNAL OPEN ACCESS FULL-TEXT HTMLDownload PDF (277K) Full view HTML -
Kodai Iwahashi, Shuji Hashimoto, Ryohei Yamauchi, Keijiro Saito, Masak ...Article type: Regular Article
2022Volume 108Issue 8 Pages 440-448
Published: 2022
Released on J-STAGE: July 31, 2022
JOURNAL OPEN ACCESS FULL-TEXT HTMLThe knowledge of Cr2O3 activity in slag is necessary to decarburize chromium-containing special steel while minimizing the oxidation loss of chromium. In the present study, firstly, the phase relationship in the CaO-SiO2-Cr2O3 system was determined at 1573 K. Subsequently, the Cr2O3 activities in two- and three-phase coexisting slags at 1573 K were measured by equilibrating molten copper with oxide phases under a stream of Ar + H2 + CO2 gas mixtures, and the Gibbs energy changes of the formations of CaCr2O4 and Ca3Cr2Si3O12 were derived. It was found that the present results were compatible with the phase diagram and the literature data reported at higher temperature than 1573 K.
 View full abstractDownload PDF (2150K) Full view HTML
View full abstractDownload PDF (2150K) Full view HTML -
Zhi Li, Chisei Kato, Yoshinao KobayashiArticle type: Regular Article
2022Volume 108Issue 8 Pages 449-454
Published: 2022
Released on J-STAGE: July 31, 2022
JOURNAL OPEN ACCESS FULL-TEXT HTMLFor a better thermodynamic understanding of the optimization of molten slag in the stainless-steel refining process, the solubility of chromium oxide in the CaO–SiO2–Cr2O3 slag system was investigated. A chemical equilibrium technique was employed in which molten slag and solid Cr2O3 pellets were equilibrated under a regulated oxygen partial pressure (PO2) lower than 10−11 atm at 1873 K. The mass ratio of CaO to SiO2 (XCaO/XSiO2), as an index of slag basicity, was varied from 0.5 to 1.5. A CO–CO2–Ar gas mixture was used, and the oxygen partial pressure was precisely controlled by evaluating possible contamination by oxygen that was inevitably introduced into the gas mixture system. A zirconia oxygen sensor was used to directly measure the oxygen partial pressure for this evaluation. The solubility of chromium oxide increased with decreasing slag basicity and oxygen partial pressure. Accordingly, slag design is a huge prospect toward achieving desirable refining conditions.
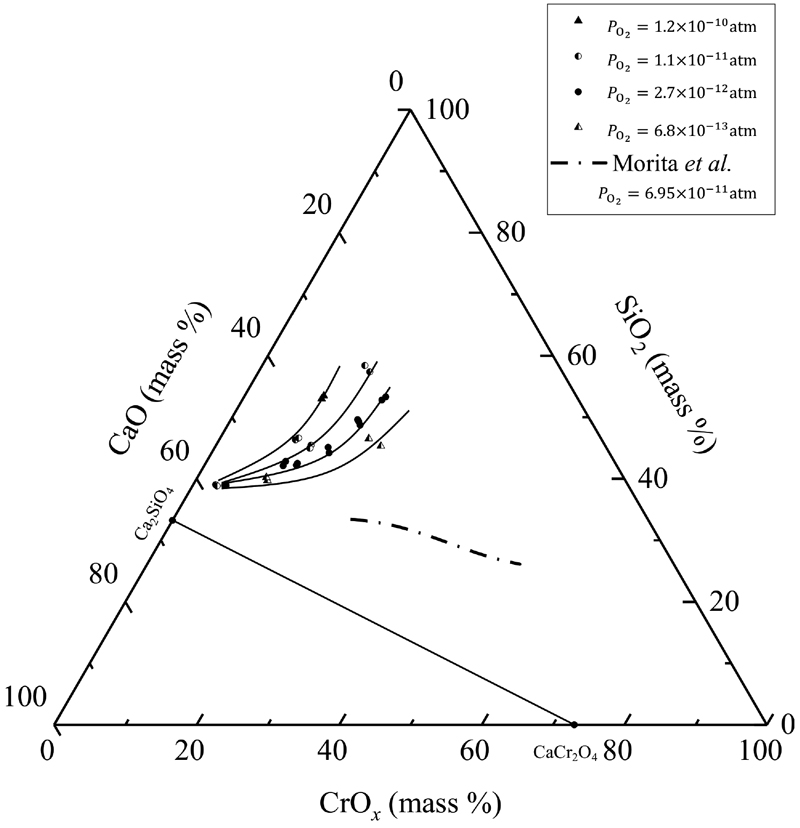 View full abstractDownload PDF (1155K) Full view HTML
View full abstractDownload PDF (1155K) Full view HTML -
Kozo Shinoda, Sohei SukenagaArticle type: Regular Article
2022Volume 108Issue 8 Pages 455-460
Published: 2022
Released on J-STAGE: July 31, 2022
JOURNAL OPEN ACCESS FULL-TEXT HTMLThe chemical states of Fe and Cr in CaO-SiO2-MgO based oxide glasses prepared assuming actual chromium steel slag with different compositions and different melting conditions were investigated using X-ray absorption near edge structure (XANES) measured by X-ray absorption spectroscopy (XAS), and the valence ratios of each metal were determined. The results showed that the valence ratios changed depending on the oxygen partial pressure during melting, the basicity (CaO/SiO2 weight ratio), and the coexistence of Fe and Cr. It was also found that Fe was a two-component system with divalent and trivalent valences coexisting, while Cr had divalent, trivalent, and hexavalent valences, but only divalent and trivalent or trivalent and hexavalent valences coexisted. In particular, the effect of coexistence is complicated by the chemical states of Fe and Cr. The effect of MgO addition is not as large as that of metal oxide coexistence, but it is not only as a basic oxide. The usefulness of direct and quantitative analysis of metal chemical states by XAS was demonstrated.
 View full abstractDownload PDF (848K) Full view HTML
View full abstractDownload PDF (848K) Full view HTML -
Yoshihiko Higuchi, Hideki Ono, Katsuyoshi OkumotoArticle type: Review
2022Volume 108Issue 8 Pages 460-468
Published: 2022
Released on J-STAGE: July 31, 2022
Advance online publication: March 04, 2022JOURNAL OPEN ACCESS FULL-TEXT HTMLControlling inclusion content in high chromium steel is very important to prevent submerged entry nozzle from clogging in continuous casting and avoid the negative impacts of inclusions on steel properties. Therefore, effects of temperature and content of elements on phase stability diagram should be clarified in chromium bearing steel. However, the effect of chromium content on boundaries of MgO, MgO∙Al2O3 and Al2O3 in phase stability diagram are much different among the researchers. The direction of boundaries shift is affected by chromium content differently. Temperature dependencies of deoxidation equilibrium constants below 1873 K are also scattered. Calcium, which is used to avoid the negative effect of MgO∙Al2O3 inclusion, enlarges liquid region in phase stability diagram. However, the region replaced by liquid oxide is understood differently in low alloyed steel and high chromium steel. In TiOx-Al2O3-MgO system inclusion, commercial thermochemical software predicts that boundaries of Ti2O3, Ti3O5, Al2O3 and TiOx-Al2O3 shift toward lower titanium content in high chromium steel. However, the calculated phase stability diagrams vary among studies even in liquid iron or low alloyed steel. Therefore, equilibrium experiments under various conditions and reliable technique of thermodynamic calculation with high accuracy are desired.
 View full abstractDownload PDF (3902K) Full view HTML
View full abstractDownload PDF (3902K) Full view HTML -
Hiroshi Fukaya, Seika Nakajima, Jonah Gamutan, Shigeru Suzuki, Koji Ka ...Article type: Regular Article
2022Volume 108Issue 8 Pages 469-478
Published: 2022
Released on J-STAGE: July 31, 2022
JOURNAL OPEN ACCESS FULL-TEXT HTMLThe aluminum deoxidation equilibrium in molten Fe-10 to 40 mass%Cr-8 mass%Ni and Fe-18 mass%Cr-8 to 30 mass%Ni alloys was experimentally determined at 1 873 K and 1 773 K to obtain the thermodynamic parameters at both temperatures, corresponding to the refining and casting processes, respectively. Thermodynamic analysis on Al deoxidation was carried out based on the sub-regular solution model using a Redlich–Kister type polynomial. Fe–Al, Ni–Al, Cr–Al and Fe–Cr–Ni interaction parameters were obtained from experimental results and a thermodynamic assessment. Using these parameters, the Al deoxidation equilibrium over the complete composition range of the Fe–Ni alloy and in more than 50 mass%Fe of the Fe–Cr and Fe–Cr–Ni alloys can be calculated for the temperature ranges of both of the refining and casting processes.
 View full abstractDownload PDF (2281K) Full view HTML
View full abstractDownload PDF (2281K) Full view HTML -
Katsuyoshi Okumoto, Kengo Kato, Hideki Ono, Yoshihiko HiguchiArticle type: Regular Article
2022Volume 108Issue 8 Pages 479-490
Published: 2022
Released on J-STAGE: July 31, 2022
JOURNAL OPEN ACCESS FULL-TEXT HTMLDemands for cleanliness of high chromium steel have been increasing. In steel refining process, aluminum is usually added in molten steel as a deoxidizing agent. As a result, such inclusions as alumina (Al2O3) and spinel (MgO∙Al2O3) are formed, which cause fatigue failures and surface defects. Therefore, it is important to understand the conditions of the inclusions which form in high chromium steel, and to reduce their harmful effects on steel qualities. In this work, to begin with, thermodynamic conditions of MgO and MgO∙Al2O3 formation in Fe-17 mass%Cr molten steel at 1873 K were investigated. The results showed that MgO is more stable in high chromium steel than in plain steel. The boundary of the stable condition of MgO and MgO∙Al2O3 shifts toward higher Al and lower Mg contents in high Cr steel. This cause is judged to be the effect of thermodynamic interaction between Cr and Mg. The interaction parameter of Cr on Mg was estimated to be 0.040 so that the boundary of stable condition of MgO and MgO∙Al2O3 can be explained. Moreover, phase stability diagram of Fe-Cr-Al-Ca-Mg-O system at 1873 K was developed to estimate the effect of chromium on the stable condition of MgO, MgO∙Al2O3 and CaO-MgO-Al2O3(l). Subsequently, the variations of inclusions which formed in Fe-17 mass%Cr molten steel were also investigated at 1873 K. The variations of inclusions in molten Fe-Cr steel were reasonably explained by considering the stable conditions of MgO and MgO∙Al2O3 investigated in this work.
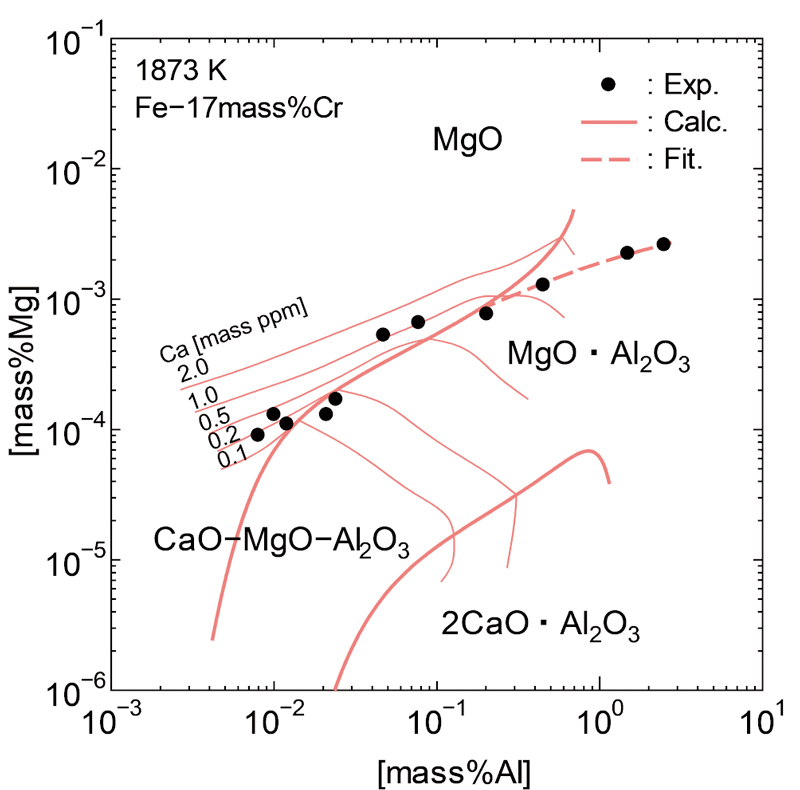 View full abstractDownload PDF (5835K) Full view HTML
View full abstractDownload PDF (5835K) Full view HTML -
Ying Ren, Chunyang Liu, Xu Gao, Lifeng Zhang, Shigeru Ueda, Shin-ya Ki ...Article type: Regular Article
2022Volume 108Issue 8 Pages 491-500
Published: 2022
Released on J-STAGE: July 31, 2022
JOURNAL OPEN ACCESS FULL-TEXT HTMLDolomite refractories are widely used in the refining process of clean steel and are considered potential sources of Mg and Ca that form MgO·Al2O3 spinel and CaO-containing inclusions. In this study, dolomite refractories were immersed into Al-killed molten steel with either 0.05% Al or 0.25% Al. The dissolution behavior of Mg and Ca from the dolomite refractory was studied, and the inclusion transformation behavior was observed. The results revealed that MgO in the dolomite refractory was reduced by Al in the molten steel, and the Mg content depended on the Al content. On the contrary, CaO barely dissolved into the molten steel even though the Al content increased. After immersion in both the low Al (0.05% Al) and high Al (0.25% Al) steels, an interfacial layer consisting of solid MgO and liquid phase CaO–Al2O3–MgO was formed on the surface of the rods. The initial Al2O3 inclusions gradually changed into Al2O3 saturated MgO–Al2O3 spinel after 60 min in low-Al steel; but were quickly transformed into MgO-saturated MgO–Al2O3 spinel in high Al steel. No CaO-containing inclusions were detected in the molten steel regardless of the immersion time and Al content.
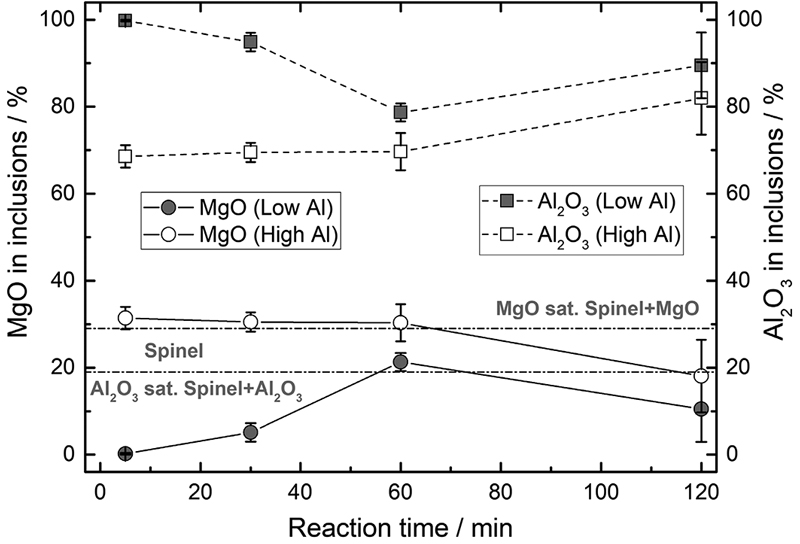 View full abstractDownload PDF (6455K) Full view HTML
View full abstractDownload PDF (6455K) Full view HTML -
Ryosuke Nishigaki, Hiroyuki MatsuuraArticle type: Regular Article
2022Volume 108Issue 8 Pages 501-508
Published: 2022
Released on J-STAGE: July 31, 2022
JOURNAL OPEN ACCESS FULL-TEXT HTMLDeoxidation equilibria of Fe–Mn–Al melt with Al2O3 or MnAl2O4 were measured at 1773 K. Composition of melts doubly-saturated with Al2O3 and MnAl2O4 were also measured using a crucible comprising these two phases at 1873 or 1773 K. Equilibria with each solid oxide were analyzed using Wagner’s Interaction Parameter Formalism (WIPF). In the case of Al2O3 saturation, Al deoxidation curve at 1773 K was similar in shape to that at 1873 K, and the equilibrium oxygen content was approximately 1/3 of that at 1873 K. The deoxidation equilibria were reproduced using WIPF at the composition range above 0.1 mass%Al by using −0.32 as eOAl and 10−13.4 as the equilibrium constant of Al2O3 dissolution reaction, both of which were determined through analysis of measured results for Fe–(20 to 30) mass% Mn melt. In the case of MnAl2O4 saturation, accurate values of equilibrium constant were not obtained because of the relatively significant influence of oxygen analysis error. On the contrary, using compositions doubly-saturated with Al2O3 and MnAl2O4, valid values of the equilibrium constant of MnAl2O4 dissolution reaction, 10−15.4 and 10−17.7 at 1873 and 1773 K, respectively, could be determined.
 View full abstractDownload PDF (2562K) Full view HTML
View full abstractDownload PDF (2562K) Full view HTML -
Jonah Gamutan, Takahiro Miki, Tetsuya NagasakaArticle type: Regular Article
2022Volume 108Issue 8 Pages 509-516
Published: 2022
Released on J-STAGE: July 31, 2022
JOURNAL OPEN ACCESS FULL-TEXT HTMLMorphology and composition of inclusions change with temperature. However, besides the temperature conditions during steelmaking or continuous casting, other factors contributing to changes in the morphology and composition of inclusions during solidification are still unknown. In this study, the formation of complex inclusions in Si-Mn deoxidized steel after isothermal holding at the solid-liquid equilibrium temperature (TS) of steel was investigated.
The typical inclusions found in the alloy were MnO-SiO2 based, spherically shaped and homogeneously distributed. With isothermal holding at the solid-liquid equilibrium temperature of steel, formation of a secondary SiO2-rich inclusion phase occurred. The changes in the composition of the inclusions depended on the manganese and silicon contents in the metal.
The general mechanism of inclusion formation observed in this study can be divided into three steps: 1) the formation of primary MnO-SiO2 inclusions above the liquidus temperature when the steel is in a completely molten state as a result of the deoxidation process; 2) the nucleation of secondary inclusions as the molten steel becomes supersaturated with the solute elements while holding at the solid-liquid equilibrium temperature; and 3) the growth and coalescence of inclusions due to natural convection in the molten alloy. From this, the inclusions formed in Si-Mn deoxidized alloys held isothermally at the solid-liquid equilibrium temperature were of three types: primary MnO-SiO2 inclusions, secondary SiO2 inclusions and complex inclusions with both primary MnO-SiO2 inclusions and precipitated secondary SiO2 inclusions.
 View full abstractDownload PDF (1431K) Full view HTML
View full abstractDownload PDF (1431K) Full view HTML -
Jonah Gamutan, Takahiro Miki, Tetsuya NagasakaArticle type: Regular Article
2022Volume 108Issue 8 Pages 517-524
Published: 2022
Released on J-STAGE: July 31, 2022
JOURNAL OPEN ACCESS FULL-TEXT HTMLWith the emerging significance of creating an acicular ferrite microstructure to provide an optimum set of properties in steel, MnS precipitation behavior on a MnO-SiO2 inclusion at the solid-liquid coexistence temperature was experimentally investigated and thermodynamically elucidated in this study. Using a direct method of forming inclusions, alloy samples with varying sulfur concentrations [Fe-1.1Mn-0.10Si-0.05O-S (initial mass %); 0.005 to 0.031 initial mass % S] were prepared by holding at the solid-liquid coexistence temperature for 1 hour.
In samples with less than 0.011 mass % sulfur, the formation of a MnO-SiO2 inclusion with a SiO2-rich precipitate was observed. Formation of SiO2 was described as a consequence of silicon enrichment in the liquid phase, which, under appropriate thermodynamic conditions, homogeneously precipitated and later on coalesced with the primary MnO-SiO2 phase. On the other hand, in samples with more than 0.022 mass % sulfur, heterogeneous precipitation of MnS along the boundary of the primary MnO-SiO2 inclusion and the alloy matrix was observed. Also, the SiO2-rich phase was found to disappear with increasing sulfur addition. Since the likelihood of heterogeneous nucleation is higher than homogeneous nucleation, it was assumed that MnS precipitation on the surface of the primary MnO-SiO2 and prevented the secondary SiO2-rich inclusion from coalescing with the existing MnO-SiO2 inclusion. This was also further validated for solute enrichment in the liquid phase, wherein MnS precipitation temperature was found to shift to higher temperatures in alloys with higher sulfur content.
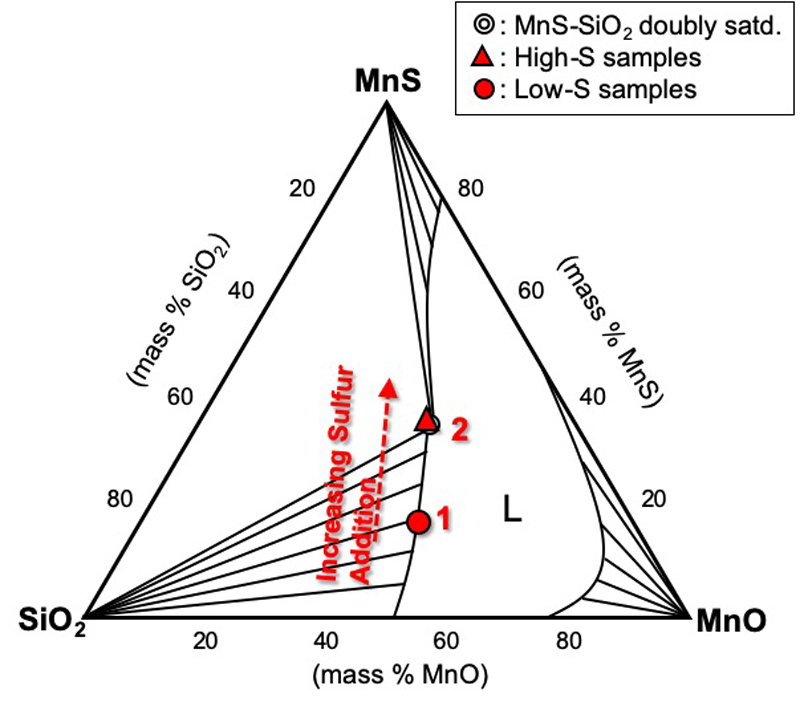 View full abstractDownload PDF (1670K) Full view HTML
View full abstractDownload PDF (1670K) Full view HTML -
Yan Lu, Takahiro MikiArticle type: Regular Article
2022Volume 108Issue 8 Pages 525-534
Published: 2022
Released on J-STAGE: July 31, 2022
JOURNAL OPEN ACCESS FULL-TEXT HTMLPhase equilibria in Fe-Mn-S and Fe-Cr-S ternary systems at 1843 K were investigated experimentally, respectively. The main characteristic of these two systems at 1843 K was confirmed to be a wide miscibility gap between two liquid phases: molten metal alloy phase and molten sulfide phase. Through metal/sulfide equilibrium method, activity of constituents in sulfide phase were determined in Fe-Mn-S and Fe-Cr-S systems separately. The activity curves of constituents in sulfide phase were estimated by utilizing regular solution model.
 View full abstractDownload PDF (3073K) Full view HTML
View full abstractDownload PDF (3073K) Full view HTML -
Yan Lu, Takahiro MikiArticle type: Regular Article
2022Volume 108Issue 8 Pages 535-540
Published: 2022
Released on J-STAGE: July 31, 2022
JOURNAL OPEN ACCESS FULL-TEXT HTMLPhase equilibria in Fe-Cr-Mn-S quaternary system at 1843 K were investigated experimentally. Two liquid phases: molten metal alloy phase and molten sulfide phase were in equilibrium in this system at 1843 K. The equilibrium relations between molten metal alloy and sulfide phases were experimentally measured. By using metal/sulfide equilibrium method, activity of constituents in molten MnS-CrS-FeS sulfide phase were determined. By utilizing regular solution model, activity curves of constituents in sulfide phase were estimated.
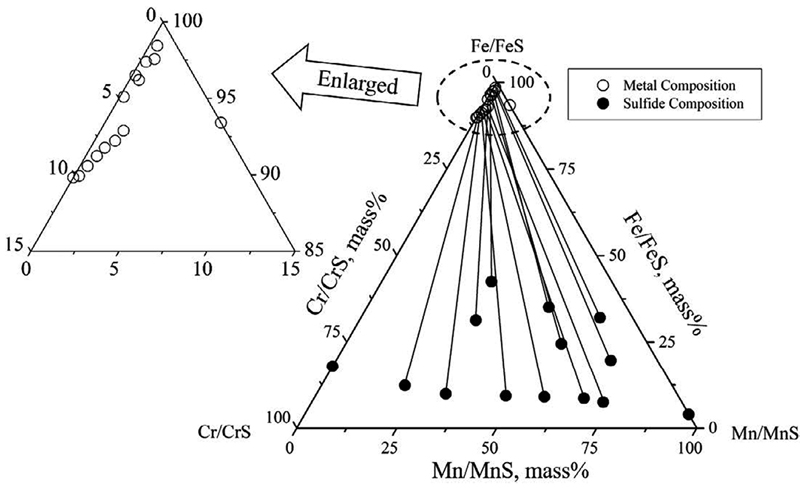 View full abstractDownload PDF (1605K) Full view HTML
View full abstractDownload PDF (1605K) Full view HTML -
Yan Lu, Takahiro MikiArticle type: Regular Article
2022Volume 108Issue 8 Pages 541-551
Published: 2022
Released on J-STAGE: July 30, 1960
JOURNAL OPEN ACCESS FULL-TEXT HTMLThermodynamic property of solid MnS-CrS-FeS system was determined based on the combination between thermodynamic properties of liquid MnS-FeS, CrS-FeS, and MnS-CrS-FeS phase determined by the authors and reported phase diagram of MnS-FeS, CrS-FeS and MnS-CrS system. The determined parameters were verified by comparison with experimental results of equilibrium relationship between metal/sulfide in Fe-Cr-Mn-S system at 1793K. By utilizing the determined parameters, phase equilibria involving sulfide phase in liquid Fe-Cr-Mn-S system was established. According to the phase equilibria information controllability of MnS-CrS-FeS sulfide phase in typical stainless steel during solidification was evaluated.
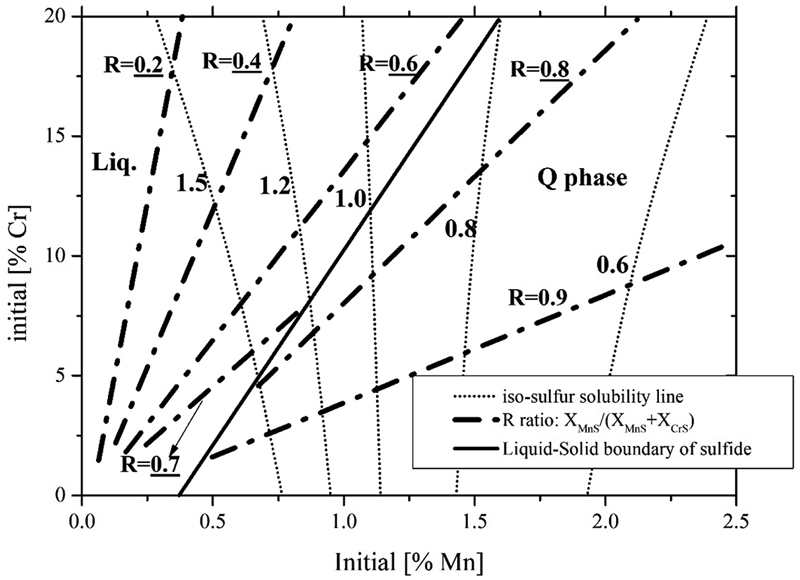 View full abstractDownload PDF (3463K) Full view HTML
View full abstractDownload PDF (3463K) Full view HTML -
Yuto Sakaizawa, Shigeru Kaneko, Yasuhiro Ehara, Shigeo FukumotoArticle type: Regular Article
2022Volume 108Issue 8 Pages 552-559
Published: 2022
Released on J-STAGE: July 31, 2022
JOURNAL OPEN ACCESS FULL-TEXT HTMLThe mechanism of monotectic sulfide formation in free-machining ferritic stainless steel (SUS430F) was investigated. Monotectic sulfides were observed in SUS430F ingots with Si-Mn deoxidation. Eutectic sulfide was found in Al deoxidized ingot. The selection of sulfide morphology can be predicted by the thermodynamic calculation using FactSage software. It was thought that the decrease in sulfide activity by dissolved oxygen as an oxy-sulfide was contributed to the monotectic sulfide formation. Microsegregation of sulfur and oxygen during solidification reduces the interfacial energy between liquid and sulfides and promotes the nucleation of sulfides. Thermodynamic stability was considered to determine the selection of sulfide morphology in SUS430F.
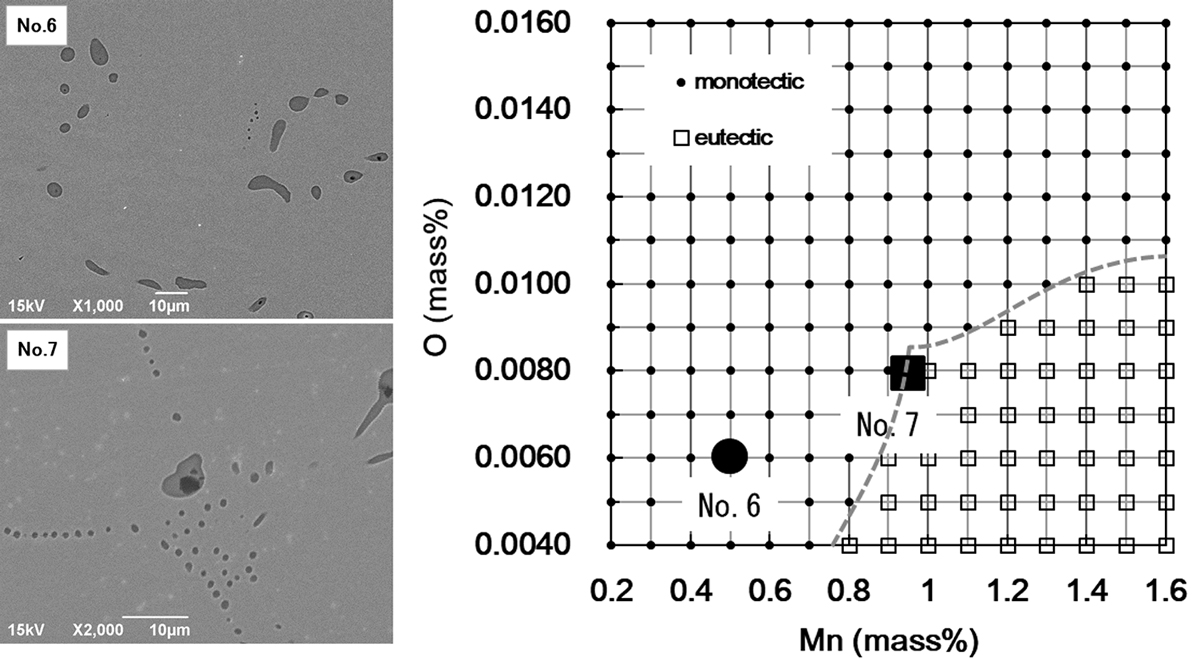 View full abstractDownload PDF (4280K) Full view HTML
View full abstractDownload PDF (4280K) Full view HTML -
Tomoki Furukawa, Noritaka Saito, Kunihiko NakashimaArticle type: Regular Article
2022Volume 108Issue 8 Pages 560-571
Published: 2022
Released on J-STAGE: July 31, 2022
Advance online publication: March 30, 2022JOURNAL OPEN ACCESS FULL-TEXT HTMLThe contact angles between three non-metallic inclusion-type oxide substrates, viz. Al2O3, MgO, and MgO·Al2O3, and molten Fe and molten Fe-based stainless steel (Fe-Cr-Ni alloy) were measured using the sessile drop method in Ar atmosphere at 1873 K. The contact angles between molten Fe and oxide substrates ranged between 111° and 117°, while that between molten Fe-Cr-Ni alloy and substrates ranged between 103° and 105°. The angles between the alloy and each of the substrates were smaller than the corresponding values for Fe, which was attributed to the superior wettability of molten Fe-Cr-Ni alloy on the substrates. The wettability of the molten materials is related to the interfacial tension between the molten metals and each substrate. Thus, the interfacial tension between the molten metals and the non-metallic substrates was quantitatively evaluated using Young’s equation and the measured contact angles; the interfacial tension for molten Fe ranged from 1.862 to 2.781 N·m−1, while that for molten Fe-Cr-Ni alloy ranged from 1.513 to 2.286 N·m−1. Owing to the higher reactivity between molten Fe-Cr-Ni alloy and the substrates, the interfacial tension and energy between them were lower than those between molten Fe and the substrates.
 View full abstractDownload PDF (4841K) Full view HTML
View full abstractDownload PDF (4841K) Full view HTML -
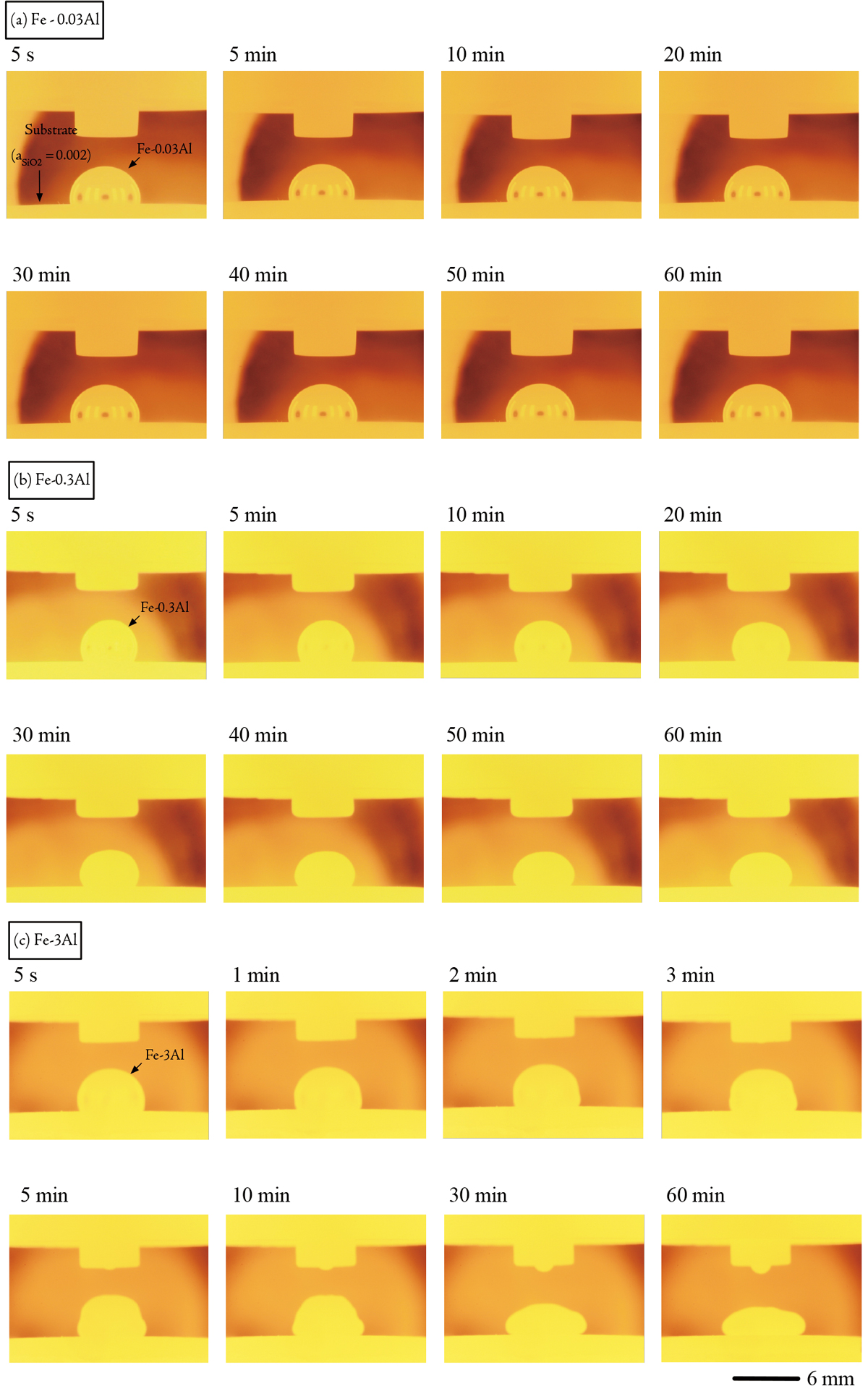
Tomoki Furukawa, Ziyao Zhang, Taro Hirosumi, Noritaka Saito, Kunihiko ...Article type: Regular Article
2022Volume 108Issue 8 Pages 572-583
Published: 2022
Released on J-STAGE: July 31, 2022
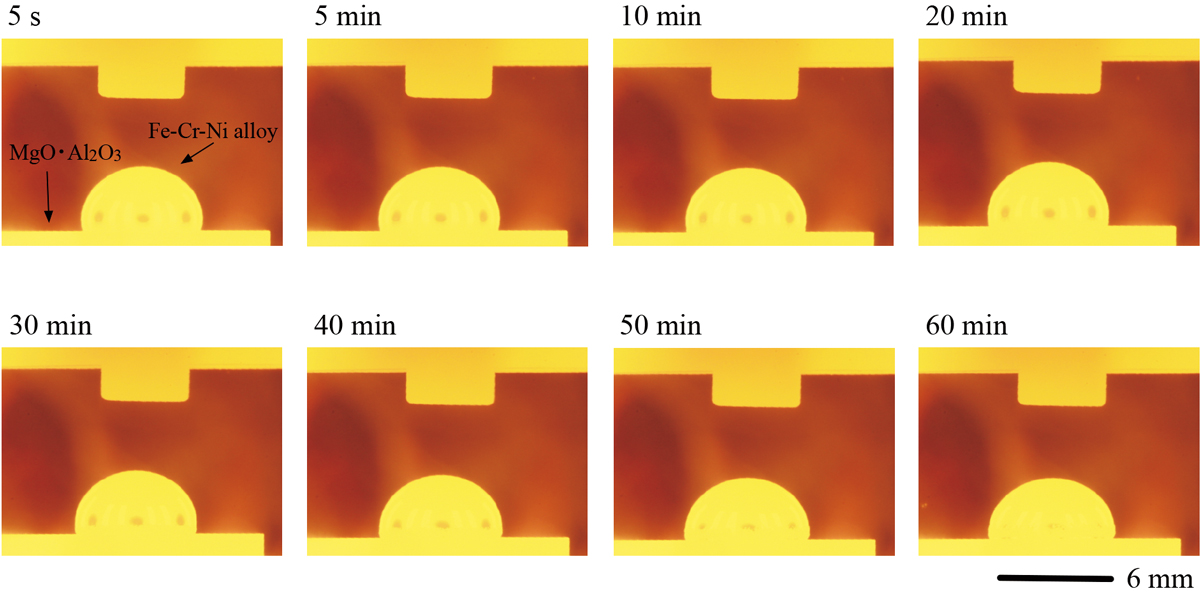
Advance online publication: March 30, 2022JOURNAL OPEN ACCESS FULL-TEXT HTMLThe contact angle between molten Fe-Al alloy with 0.03, 0.3, and 3 mass% Al composition, and Y2O3 matrix oxide substrate with 0.002, 0.32, and 1 SiO2 activity was measured using sessile drop method in Ar atmosphere at 1873 K, and the interfacial tension was evaluated. The contact angle and interfacial tension between the molten Fe-0.3 Al alloy and the Y2Si2O7 + SiO2 (aSiO2 = 1) substrate decreased over time during 60 s after the molten alloy was dropped onto the substrate. The decrease of the contact angle was 20°, and that of the interfacial tension was 628 mN・m−1 Conversely, the other contact angles and the other interfacial energies were almost stable during the same period. The decrease of the contact angles ranged between 0° and 7°, and that of the interfacial tensions ranged 4 and 195 mN・m−1. By observing the wetting behavior for 60 min, it was recognized that the interfacial reaction between the Fe-Al alloy and the oxide substrate was the redox reaction between Al composition in the alloy and SiO2 composition in the substrate, composed of SiO2 decomposition reaction and Al2O3 formation reaction between oxygen absorbed at the interface and Al composition in the alloy. In addition, it was indicated from the interfacial tension dependence on SiO2 activity that the medium SiO2 volume slag for the molten low-Al steel and the low SiO2 volume slag for the molten high-Al steel were effective in preventing the small droplets of molten slag into the molten steel.
 View full abstractDownload PDF (4982K) Full view HTML
View full abstractDownload PDF (4982K) Full view HTML
- |<
- <
- 1
- >
- >|