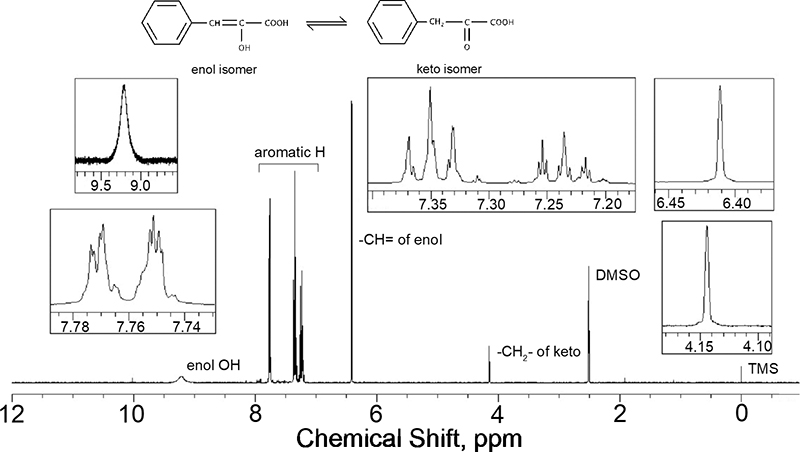
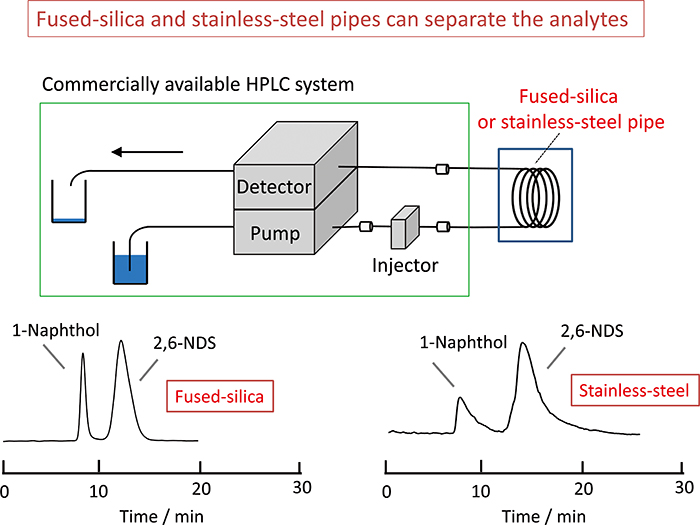
Volume 34, Issue 2
Displaying 1-22 of 22 articles from this issue
- |<
- <
- 1
- >
- >|
Highlights
-
Article type: Highlights
2018Volume 34Issue 2 Pages 125-126
Published: February 10, 2018
Released on J-STAGE: February 10, 2018
Download PDF (449K)
Rapid Communications
-
Article type: Rapid Communications
2018Volume 34Issue 2 Pages 127-130
Published: February 10, 2018
Released on J-STAGE: February 10, 2018
Download PDF (928K)
Original Papers
-
Article type: Original Papers
2018Volume 34Issue 2 Pages 131-136
Published: February 10, 2018
Released on J-STAGE: February 10, 2018
Download PDF (2363K) -
Article type: Original Papers
2018Volume 34Issue 2 Pages 137-142
Published: February 10, 2018
Released on J-STAGE: February 10, 2018
Download PDF (1760K) -
Article type: Original Papers
2018Volume 34Issue 2 Pages 143-148
Published: February 10, 2018
Released on J-STAGE: February 10, 2018
Download PDF (845K) -
Article type: Original Papers
2018Volume 34Issue 2 Pages 149-153
Published: February 10, 2018
Released on J-STAGE: February 10, 2018
Download PDF (811K) -
Article type: Original Papers
2018Volume 34Issue 2 Pages 155-160
Published: February 10, 2018
Released on J-STAGE: February 10, 2018
Download PDF (1611K) -
Article type: Original Papers
2018Volume 34Issue 2 Pages 161-167
Published: February 10, 2018
Released on J-STAGE: February 10, 2018
Download PDF (1310K) -
Article type: Original Papers
2018Volume 34Issue 2 Pages 169-175
Published: February 10, 2018
Released on J-STAGE: February 10, 2018
Download PDF (1937K) -
Article type: Original Papers
2018Volume 34Issue 2 Pages 177-182
Published: February 10, 2018
Released on J-STAGE: February 10, 2018
Download PDF (585K) -
Article type: Original Papers
2018Volume 34Issue 2 Pages 183-187
Published: February 10, 2018
Released on J-STAGE: February 10, 2018
Download PDF (1456K) -
Article type: Original Papers
2018Volume 34Issue 2 Pages 189-193
Published: February 10, 2018
Released on J-STAGE: February 10, 2018
Download PDF (607K) -
Article type: Original Papers
2018Volume 34Issue 2 Pages 195-199
Published: February 10, 2018
Released on J-STAGE: February 10, 2018
Download PDF (893K) -
Article type: Original Papers
2018Volume 34Issue 2 Pages 201-205
Published: February 10, 2018
Released on J-STAGE: February 10, 2018
Download PDF (714K) -
Article type: Original Papers
2018Volume 34Issue 2 Pages 207-214
Published: February 10, 2018
Released on J-STAGE: February 10, 2018
Download PDF (1348K) -
Article type: Original Papers
2018Volume 34Issue 2 Pages 215-220
Published: February 10, 2018
Released on J-STAGE: February 10, 2018
Download PDF (2100K) -
Article type: Original Papers
2018Volume 34Issue 2 Pages 221-225
Published: February 10, 2018
Released on J-STAGE: February 10, 2018
Download PDF (1141K) -
Article type: Original Papers
2018Volume 34Issue 2 Pages 227-233
Published: February 10, 2018
Released on J-STAGE: February 10, 2018
Download PDF (1740K)
Notes
-
Article type: Notes
2018Volume 34Issue 2 Pages 235-237
Published: February 10, 2018
Released on J-STAGE: February 10, 2018
Download PDF (549K) -
Article type: Notes
2018Volume 34Issue 2 Pages 239-241
Published: February 10, 2018
Released on J-STAGE: February 10, 2018
Download PDF (559K)
Advancements in Instrumentation
-
Article type: Advancements in Instrumentation
2018Volume 34Issue 2 Pages 243-247
Published: February 10, 2018
Released on J-STAGE: February 10, 2018
Download PDF (567K)
Announcements
-
Article type: Announcements
2018Volume 34Issue 2 Pages 249
Published: February 10, 2018
Released on J-STAGE: February 10, 2018
Download PDF (3221K)
- |<
- <
- 1
- >
- >|