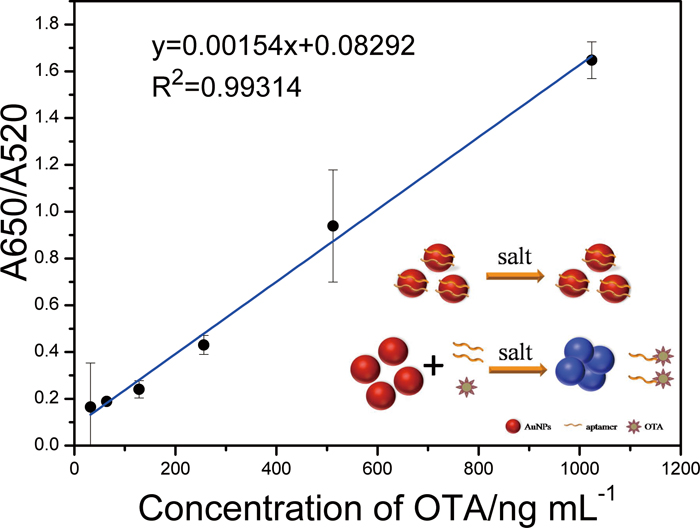
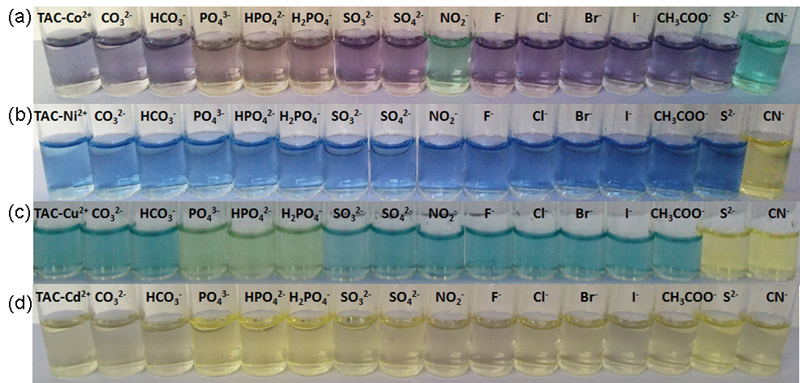
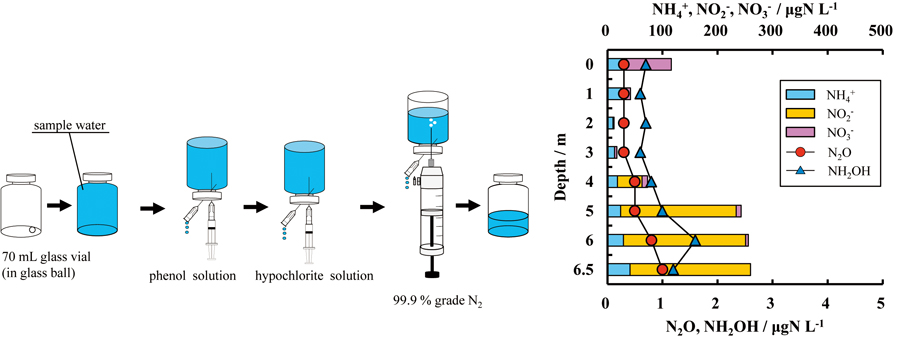
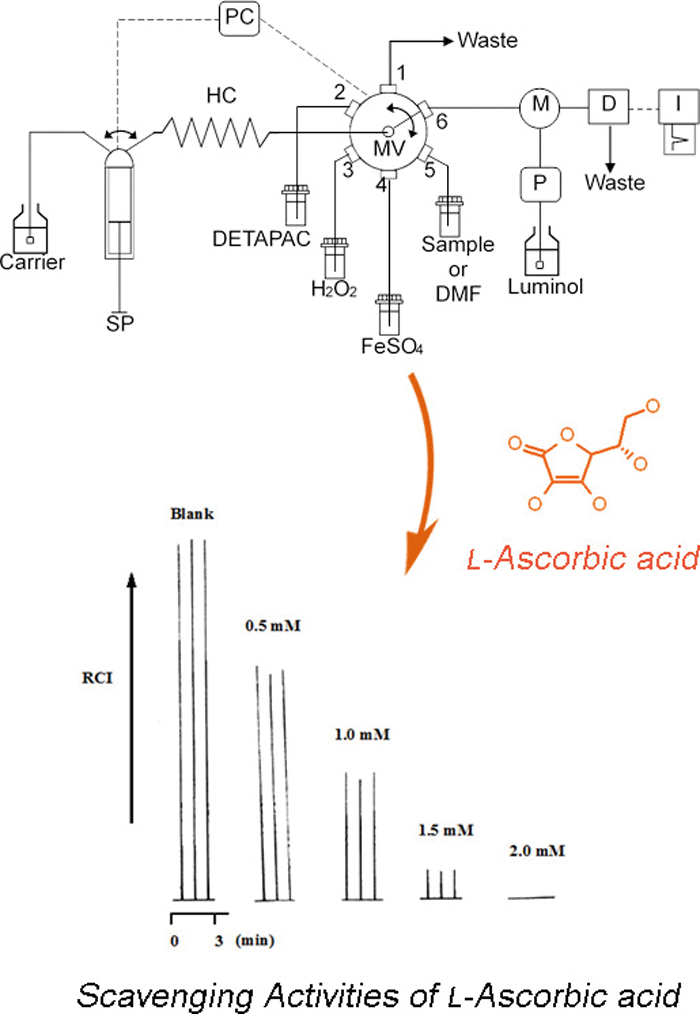
Volume 33, Issue 6
Displaying 1-19 of 19 articles from this issue
- |<
- <
- 1
- >
- >|
-
2017Volume 33Issue 6 Pages 657-658
Published: June 10, 2017
Released on J-STAGE: June 10, 2017
Download PDF (892K)
Original Papers
-
Article type: Original Papers
2017Volume 33Issue 6 Pages 659-664
Published: June 10, 2017
Released on J-STAGE: June 10, 2017
Download PDF (3144K) -
Article type: Original Papers
2017Volume 33Issue 6 Pages 665-670
Published: June 10, 2017
Released on J-STAGE: June 10, 2017
Download PDF (1051K) -
Article type: Original Papers
2017Volume 33Issue 6 Pages 671-675
Published: June 10, 2017
Released on J-STAGE: June 10, 2017
Download PDF (1074K) -
Article type: Original Papers
2017Volume 33Issue 6 Pages 677-682
Published: June 10, 2017
Released on J-STAGE: June 10, 2017
Download PDF (3523K) -
Article type: Original Papers
2017Volume 33Issue 6 Pages 683-689
Published: June 10, 2017
Released on J-STAGE: June 10, 2017
Download PDF (793K) -
Article type: Original Papers
2017Volume 33Issue 6 Pages 691-695
Published: June 10, 2017
Released on J-STAGE: June 10, 2017
Download PDF (1161K) -
Article type: Original Papers
2017Volume 33Issue 6 Pages 697-701
Published: June 10, 2017
Released on J-STAGE: June 10, 2017
Download PDF (516K) -
Article type: Original Papers
2017Volume 33Issue 6 Pages 703-707
Published: June 10, 2017
Released on J-STAGE: June 10, 2017
Download PDF (990K) -
Article type: Original Papers
2017Volume 33Issue 6 Pages 709-713
Published: June 10, 2017
Released on J-STAGE: June 10, 2017
Download PDF (596K)
Notes
-
Article type: Notes
2017Volume 33Issue 6 Pages 715-717
Published: June 10, 2017
Released on J-STAGE: June 10, 2017
Download PDF (1475K) -
Article type: Notes
2017Volume 33Issue 6 Pages 719-722
Published: June 10, 2017
Released on J-STAGE: June 10, 2017
Download PDF (513K) -
Article type: Notes
2017Volume 33Issue 6 Pages 723-725
Published: June 10, 2017
Released on J-STAGE: June 10, 2017
Download PDF (585K) -
Article type: Notes
2017Volume 33Issue 6 Pages 727-730
Published: June 10, 2017
Released on J-STAGE: June 10, 2017
Download PDF (612K) -
Article type: Notes
2017Volume 33Issue 6 Pages 731-733
Published: June 10, 2017
Released on J-STAGE: June 10, 2017
Download PDF (451K) -
Article type: Notes
2017Volume 33Issue 6 Pages 735-738
Published: June 10, 2017
Released on J-STAGE: June 10, 2017
Download PDF (725K) -
Article type: Notes
2017Volume 33Issue 6 Pages 739-742
Published: June 10, 2017
Released on J-STAGE: June 10, 2017
Download PDF (665K) -
Article type: Notes
2017Volume 33Issue 6 Pages 743-746
Published: June 10, 2017
Released on J-STAGE: June 10, 2017
Download PDF (515K)
Announcements
-
Article type: Announcements
2017Volume 33Issue 6 Pages 747
Published: June 10, 2017
Released on J-STAGE: June 10, 2017
Download PDF (3037K)
- |<
- <
- 1
- >
- >|