All issues

Volume 33, Issue 10
Displaying 1-19 of 19 articles from this issue
- |<
- <
- 1
- >
- >|
Highlights
-
Fumitaka ESAKAArticle type: Highlights
2017 Volume 33 Issue 10 Pages 1097-1098
Published: October 10, 2017
Released on J-STAGE: October 10, 2017
JOURNAL FREE ACCESSDownload PDF (437K)
Original Papers
-
Zhengsheng MAO, Xin WANG, Xin DI, Yangdan LIU, Yanan ZANG, Dongke MA, ...Article type: Original Papers
2017 Volume 33 Issue 10 Pages 1099-1103
Published: October 10, 2017
Released on J-STAGE: October 10, 2017
JOURNAL FREE ACCESSIn this study, a rapid and reliable high-performance liquid chromatography–tandem mass spectrometry (HPLC-MS/MS) method for the determination of ambroxol in human plasma was developed and validated using palmatine as an internal standard (IS). Ambroxol and IS were extracted from 200 μL of human plasma via a simple protein precipitation preparation. Chromatographic separation was achieved on a Platisil C18 column (150 × 4.6 mm, 5 μm) using methanol–0.01% formic acid (70:30, v/v) as the mobile phase at a flow rate of 0.6 mL/min under an isocratic condition. The MS acquisition m/z 379 → 264 for ambroxol and 352 → 336 for IS was performed by atmospheric-pressure chemical ionization (APCI) mass spectrometry in selected reaction monitoring mode. The calibration curve for ambroxol was linear over the concentration range of 2.500 – 180.0 ng/mL. The matrix effects of ambroxol ranged from 104.6 to 112.7%. This fully validated method was successfully applied to a pharmacokinetic study of ambroxol in humans after oral administration of ambroxol at a single dose of 75 mg. View full abstractDownload PDF (726K)
View full abstractDownload PDF (726K) -
Yanxia LI, Lu HUANG, Xiuping WANG, Yiting CHENArticle type: Original Papers
2017 Volume 33 Issue 10 Pages 1105-1110
Published: October 10, 2017
Released on J-STAGE: October 10, 2017
JOURNAL FREE ACCESSA study of an electrochemical sensor based on bovine hemoglobin (BHb) imprinted magnetic nanoparticles (MIPs) was investigated. First, BHb MIPs were successfully synthesized with magnetic Fe3O4@Au nanoparticles as carrier by surface modification of mercapto propionic acid for introducing carboxyl groups, combined with dopamine as the functional monomer and BHb as the template protein. Then, the MIPs were modified to the surface of a glassy carbon electrode (GCE) for electrochemical analysis by using cyclic voltammetry in the potassium ferricyanide solution. Results show that there was a good dynamic response between electrochemical signals and the adsorption amount of protein in the range of 0.05 to 0.5 mg mL−1. Results show that the sensor exhibits a significant specific recognition toward the template protein via selective test and can be used for analysis of serum samples. The synthesized MIPs are suitable for the removal and enrichment of template protein in proteomics. At the same time, the proposed electrochemical sensor can be used for recognition of BHb. View full abstractDownload PDF (2000K)
View full abstractDownload PDF (2000K) -
Daniela M. BATISTELA, Cassius V. STEVANI, Renato S. FREIREArticle type: Original Papers
2017 Volume 33 Issue 10 Pages 1111-1114
Published: October 10, 2017
Released on J-STAGE: October 10, 2017
JOURNAL FREE ACCESSA simple colorimetric immunoassay for quantification of human immunoglobulin G (hIgG) is herein described. The assay is based on the aggregation inhibition of silver nanoparticles (AgNP) functionalized with hIgG antibody (anti-hIgG) on the surface. The aggregation is measured in terms of attenuance values ratio at 400 and 530 nm (A400/A530). A linear response between A400/A530 and hIgG concentration is observed in the range 25 – 200 ng mL−1, and the detection limit is estimated as 11 ng mL−1 hIgG. View full abstractDownload PDF (971K)
View full abstractDownload PDF (971K) -
Elumalai SATHEESHKUMAR, Jyisy YANG, Venkatesan SRINIVASADESIKAN, Ming- ...Article type: Original Papers
2017 Volume 33 Issue 10 Pages 1115-1121
Published: October 10, 2017
Released on J-STAGE: October 10, 2017
JOURNAL FREE ACCESSIn this work, a simple method was developed to simultaneously fabricate silver nanoparticles (AgNPs) and modify their surfaces with recognition functional groups for colorimetric detection of Cu2+ ions. To prepare the AgNPs with proper functional group on their surface for detection of Cu2+ ions, photochemical reaction was employed and a photoactive species of tyrosine (Tyr) was used to trigger the photoreduction of AgNPs, while the oxidized Tyr (TyrOx) was used to functionalize the AgNPs surface at the same time. To understand the behaviors, the prepared color AgNPs colloidal solution was characterized by UV-visible spectrometer, FT-IR spectrometer, dynamic light scattering (DLS), X-ray photoelectron spectrometer (XPS) and density functional theory (DFT). Based on DFT calculation results, TyrOx was adsorbed on the surface of AgNPs by the quinone ring and its functional group of amino acid was freely exposed to the aqueous media for rapid interaction of Cu2+ ions. Based on detection of different metal ions, TyrOx@AgNPs were selective to interact with Cu2+ ions through formation of highly stable Cu2+-TyrOx@AgNPs complexes. The evidence in formation of Cu2+-TyrOx@AgNPs complex could be obtained through the red shift of the surface plasmonic resonance (SPR) band of TyrOx@AgNPs located at 557 nm, which gives a color change from light yellow to brown color allowing visual identification of Cu2+ ions for rapid screening purposes. For quantitative analysis, a band intensity ratio of A557/(A404–A557) was constructed to correlate with the concentration of Cu2+ ions. A linear range up to 10 μM with a detection limit close to 150 nM was found. View full abstractDownload PDF (3718K)
View full abstractDownload PDF (3718K) -
Xiaohui CHEN, Jiahao YIN, Chao ZHANG, Nian LU, Zhidong CHENArticle type: Original Papers
2017 Volume 33 Issue 10 Pages 1123-1128
Published: October 10, 2017
Released on J-STAGE: October 10, 2017
JOURNAL FREE ACCESSA novel solid-state electrochemiluminescence (ECL) quenching sensor was constructed for determination of brilliant blue FCF (BB FCF). Under a simple electropolymerization step, poly(sulfosalicylic acid) (PSSA) film attached luminophore Ru(bpy)32+ was successfully formed on the surface of a glass carbon electrode [Ru(bpy)32+–PSSA/GCE], which exhibited excellent ECL behavior. A high quenching effect on the ECL signal of the Ru(bpy)32+–PSSA/GCE was obtained with the presence of low concentration of BB FCF. Moreover, the quenched ECL intensity showed a linear relation within the BB FCF concentration range of 0.5 – 7 and 7 – 10 μmol/L, with a detection limit of 57 nmol/L (S/N = 3). Besides, Ru(bpy)32+–PSSA/GCE exhibited good reproducibility and was successfully applied in the practical detection of BB FCF in peppermint candy samples. View full abstractDownload PDF (1844K)
View full abstractDownload PDF (1844K) -
Shanshan WANG, Yingyi WANG, Kuncheng YANG, Yan ZHONG, Xiaoming YANG, Z ...Article type: Original Papers
2017 Volume 33 Issue 10 Pages 1129-1134
Published: October 10, 2017
Released on J-STAGE: October 10, 2017
JOURNAL FREE ACCESS
Supplementary materialHereby, one kind of facile carbon dots (CDs) from hydroxypropylmethyl cellulose (HPMC) has been successfully provided, which obviously emitted blue fluorescence. With the related characterizations in detail, the CDs prepared here mainly consisted of C and O, owing to the functional groups of –OH and C=O on their surfaces. Likewise, the CDs also showed multiple advantages, including excellent photostability, superior dispersity and desirable stability. Moreover, the CDs were applied for sensing ciprofloxacin due to forming complexes with ciprofloxacin, thus leading to the fluorescence quenching of CDs. This proposed method was permitted to sense ciprofloxacin in a linear range of 10 nM L−1 – 90 μM L−1, suggesting that it may broaden the sensing ways for assaying ciprofloxacin. View full abstractDownload PDF (1080K)
View full abstractDownload PDF (1080K) -
Abdollah TAGHANI, Nasser GOUDARZI, Ghadamali BAGHERIAN, Mansour Arab C ...Article type: Original Papers
2017 Volume 33 Issue 10 Pages 1135-1140
Published: October 10, 2017
Released on J-STAGE: October 10, 2017
JOURNAL FREE ACCESSA rapid, simple, and sensitive technique is proposed based on a miniaturized solid-phase extraction method named mictroextraction in a packed syringe coupled with gas chromatography–mass spectrometry for the preconcentration and determination of three organochlorine pesticides. These include hexachlorobenzene, heptachlor and aldrine in aqueous samples. For the first time, the natural nano diatomite is used a sorbent. Based on this technique, 6.0 mg of the nano sorbent is inserted in a syringe between two polypropylene frits. The analytes would be adsorbed on the solid phase, and would subsequently be eluted using organic solvents. The influence of some important parameters, such as the solution pH, type and volume of the organic desorption solvent, and amount of sorbent on the extraction efficiency of the selected pesticides, is investigated. The proposed method shows good linearity in the range of 0.1 – 40.0 μg L−1, and at low limits of detection in the range of 0.02 – 0.13 μg L−1 using the selected ion-monitoring mode. The reproducibility of this method was found to be in the range of 3.5 – 11.1% for the understudied pesticides. In order to evaluate the matrix effect, the developed method is also applied to the preconcentration and determination of the selected pesticides in different water samples. View full abstractDownload PDF (1043K)
View full abstractDownload PDF (1043K) -
Norioki ABE, Nobuhiko IKIArticle type: Original Papers
2017 Volume 33 Issue 10 Pages 1141-1145
Published: October 10, 2017
Released on J-STAGE: October 10, 2017
JOURNAL FREE ACCESS
Supplementary materialUpon mixing with metal ions such as CdII, TbIII, CuII, NiII, PbII, ZnII, and CoII at pH 10.0, solutions of silver nanoparticles (AgNPs) coated with calix[4]arene-p-tetrasulfonate (CAS-AgNP) exhibited multi-coloration from yellow to orange, violet, and green, depending on the metal elements present, which allowed for visual discrimination of the ions. This is contrary to the AgNP sensors exhibiting a uniform color change from yellow to red upon binding of a receptor molecules at the surface of AgNPs to an analyte. The TEM images of the samples obtained from the resultant solution showed two regions. First, a region where CAS-AgNPs assembled on the surface of the metal hydroxides. The size of the hydroxide crystals varied from 50 to 200 nm with the type of metal element present, and roughly correlated with the extinction band of the aggregated AgNPs. Second, the amorphous region in which CAS-AgNPs dispersed randomly. The difference in the amount of the crystal region and the area seemed to lead to the multi-coloration. View full abstractDownload PDF (1252K)
View full abstractDownload PDF (1252K) -
Kanji MIYABE, Nozomu SUZUKIArticle type: Original Papers
2017 Volume 33 Issue 10 Pages 1147-1154
Published: October 10, 2017
Released on J-STAGE: October 10, 2017
JOURNAL FREE ACCESS
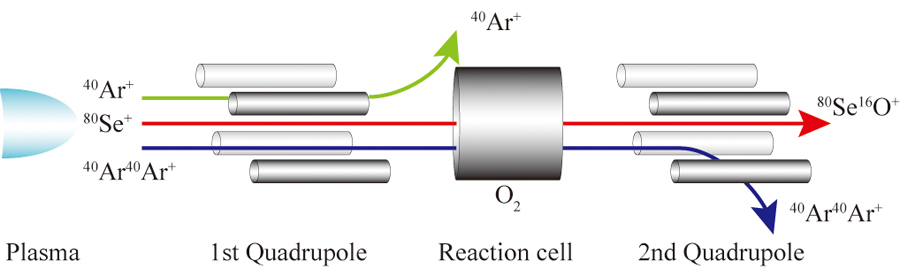
Supplementary materialNew moment equations were developed for size exclusion capillary electrochromatography (SECEC), in which intermolecular chemical reactions simultaneously took place. They explain how the first absolute and second central moments of elution peaks are correlated with some fundamental equilibrium and kinetic parameters of mass transfer and chemical reaction in SECEC column. In order to demonstrate the effectiveness of the moment equations, they were used to predict chromatographic behavior under hypothetical SECEC conditions. It was quantitatively studied how the association and dissociation rate constants of intermolecular interaction affected the position and spreading of elution peaks. It was indicated that both the intermolecular reaction kinetics and axial dispersion of solute molecules in a capillary column had a predominant contribution to the band broadening.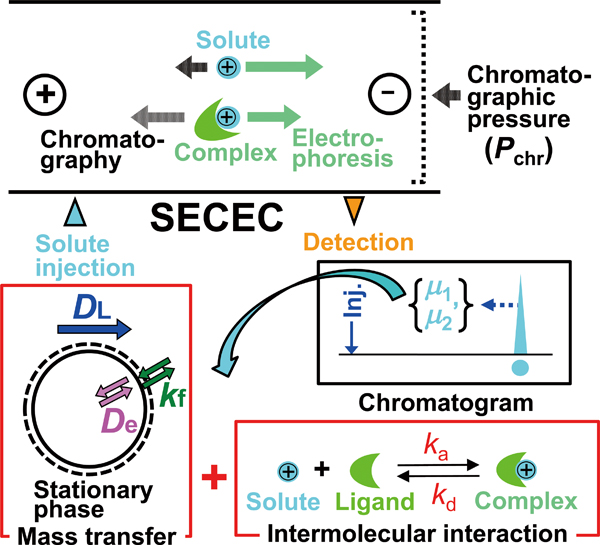 View full abstractDownload PDF (1623K)
View full abstractDownload PDF (1623K) -
Chenmeng HE, Yali TANG, Sai WANG, Jiahui LIU, Ying CHEN, Yiyang DONG, ...Article type: Original Papers
2017 Volume 33 Issue 10 Pages 1155-1160
Published: October 10, 2017
Released on J-STAGE: October 10, 2017
JOURNAL FREE ACCESSA simple and reliable electrochemical biosensor based on an in situ synthetic Au-reduced graphene oxide (Au-RGO) nanocomposite was constructed in this work, which is considered to be a potential sensing platform for sensitive and selective Bt63 rice detection. UV-vis spectroscopy, transmission electron microscopy (TEM), and cyclic voltammograms of the surface characterization indicated that RGO was prepared successfully and Au nanoparticles were well dispersed on its surface with an average size of 20 nm. The square-wave voltammetry (SWV) response of the electrochemical marker methylene blue (MB) was chosen to monitor the probe immobilization and target hybridization event. Under the optimum conditions, the peak values of MB were linear with the logarithm of the target DNA concentrations being from 1.0 × 10−9 to 1.0 × 10−14 M; the detection limit was 3.36 × 10−15 M. Studies also demonstrated that the DNA biosensor had high reproducibility and stability. At last, we used this biosensor to detect several rice samples; it showed good sensitivity and selectivity. Thus the applicability of the method as an effective tool for genetically modified organism (GMO) quantification was confirmed by its accurate and sensitive results in Bt63 rice screening.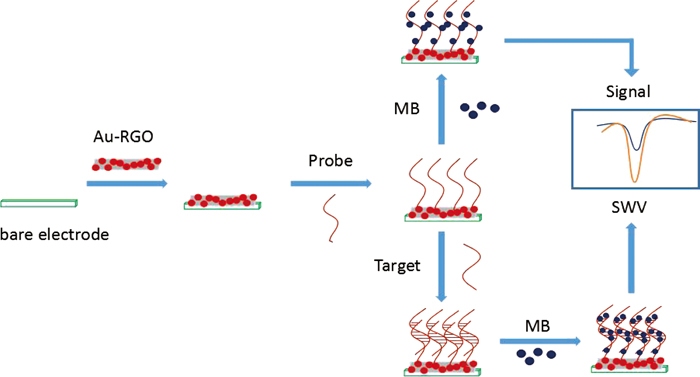 View full abstractDownload PDF (5221K)
View full abstractDownload PDF (5221K) -
Pei-Chi WU, Ewelina P. DUTKIEWICZ, Pawel L. URBANArticle type: Original Papers
2017 Volume 33 Issue 10 Pages 1161-1167
Published: October 10, 2017
Released on J-STAGE: October 10, 2017
JOURNAL FREE ACCESS
Supplementary materialThe purpose of analytical extractions is to simplify sample matrix without losing analyte molecules. Here we present a technique of extracting volatile compounds by scavenging gas-phase molecules with tiny liquid droplets (<10 μm). A cool mist of the extracting solvent is generated by an ultrasonic transducer, transferred to the headspace of the sample chamber under atmospheric conditions, and pushed by a small pressure difference toward a condenser. By slowly passing over the sample, the microdroplets extract volatile species present in the sample headspace, and they coalesce in a cooled zone. The condensed liquid is collected for analysis by direct infusion mass spectrometry or chromatography coupled with mass spectrometry. Due to the high surface-to-volume ratio of the microdroplets, the mist depletes a great share of the volatile organics present in the headspace. Other advantages of cool mist scavenging include: selective extraction of gas-phase molecules, the extracting solvent can be miscible with the sample solvent, simplicity, high speed, and no requirement for heating that could potentially decompose the sample. In this study, cool mist scavenging was first tested on artificial samples containing esters. The relationship between the sample concentration and the extract concentration was verified theoretically and experimentally. Some of the possible confounding effects were tested and discussed. The technique was subsequently applied to qualitative analysis of selected complex samples in liquid and solid phase as well as an esterification reaction. View full abstractDownload PDF (1310K)
View full abstractDownload PDF (1310K) -
Qingxia DUAN, Meng ZHANG, Chunxia SHENG, Caiyun LIU, Liu WU, Zhenmin M ...Article type: Original Papers
2017 Volume 33 Issue 10 Pages 1169-1173
Published: October 10, 2017
Released on J-STAGE: October 10, 2017
JOURNAL FREE ACCESSDeveloping some methods that can simply and effectively detect mercury ions (Hg2+) in the environment and biological systems are very important due to the problems of high toxicity and biological accumulation. Herein, we report a simple rhodol-derived colorimetric and fluorescent probe rhodol-Hg with a recognition receptor of carbonothioate for the specific determination of Hg2+. The color of probe rhodol-Hg solution changed remarkably from colorless to pink in the presence of Hg2+, thus rhodol-Hg could act as a “naked-eye” probe for Hg2+. Additionally, this probe exhibited high selectivity and ultrasensitivity in aqueous solution with the limit of detection (LOD) of 1.4 nM toward Hg2+, and the linear range was 0 – 0.8 μM determined by turn-on fluorescence spectrometry. Importantly, this probe has been successfully used for the detection of Hg2+ in environmental waters and living cells.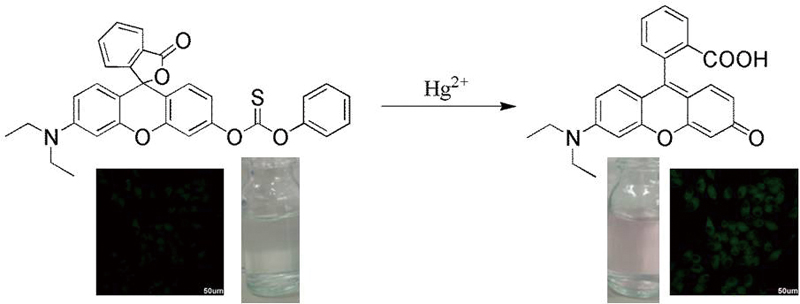 View full abstractDownload PDF (1316K)
View full abstractDownload PDF (1316K) -
Ikuo UETA, Moe ONIKATA, Koji FUJIMURA, Tomotaka YOSHIMURA, Shoji NARUK ...Article type: Original Papers
2017 Volume 33 Issue 10 Pages 1175-1180
Published: October 10, 2017
Released on J-STAGE: October 10, 2017
JOURNAL FREE ACCESS
Supplementary materialA newly designed styrene-divinylbenzene copolymer adsorbent packed solid-phase extraction (SPE)-type collection device for the quantitative determination of airborne polycyclic aromatic hydrocarbons (PAHs) containing two to five rings is reported in this manuscript. This SPE-type collection device offers rapid, easy and quantitative elution of the analytes and easier reuse. A small collection device was initially developed for investigating the basic collection and elution performances of the adsorbent with respect to PAHs. The analytes were quantitatively collected on the adsorbent up to 3 m3 of air sampling at a sampling temperature of 35°C. The collected analytes were then completely eluted from the adsorbent by passing 3 mL of dichloromethane without carry-over of the analyte. During air collection, because no moisture was trapped on the adsorbent, the subsequent gas chromatography–mass spectrometric analysis was not influenced by moisture. Based on these successful performances, a wide-bore collection device was introduced for collecting larger air samples. After a quantitative investigation of the collection and elution performances of the wide-bore collection device, it device was successfully applied for precise determinations of PAHs in atmospheric air. Further application and employment of the device for the precise determination of semi-volatile organic compounds in environmental air samples is expected due to these excellent results. View full abstractDownload PDF (700K)
View full abstractDownload PDF (700K) -
Qianqian SUN, Liu YANG, Jidong YANG, Shaopu LIU, Xiaoli HUArticle type: Original Papers
2017 Volume 33 Issue 10 Pages 1181-1187
Published: October 10, 2017
Released on J-STAGE: October 10, 2017
JOURNAL FREE ACCESSAllura red (AR) is a common food additive. It is of significance to detect AR sensitively and selectively in soft drinks. In this report, fluorescence spectra of allura red-rhodamine dyes systems were studied. In a pH 6.0 Britton–Robinson buffer medium, the fluorescence of rhodamine dyes, such as rhodamine B (RB), butylrhodamine B (BRB) and rhodamine 6G (R6G), can be quenched by AR. Impressively, the emission spectrums of the RB and BRB change slightly upon the addition of AR, but it was clear that the emission of R6G decreased dramatically in the presence of AR. Thus, we have succeeded in planning an improved method for specifically detecting AR on the basis of hydrophobic forces and the electrostatic attraction between R6G and AR. The results show that AR could combine with R6G to form an ion-association complex, which causes quenching of the emission intensity of R6G and changes of the UV-visible spectra. In this system, 0.097 – 6.0 μmol L−1 AR could be simply detected owing to the decreased fluorescence of R6G in soft drinks, with a detection limit of 0.029 μmol L−1. In addition, we also optimized the reaction conditions and evaluated the effects of some coexisting substances. According to the fluorescence decay time, the UV-visible absorption spectra and the Stern–Volmer plots, the fluorescence quenching of R6G by AR is a static quenching process. View full abstractDownload PDF (1228K)
View full abstractDownload PDF (1228K)
Notes
-
Koji FURUKAWA, Makoto HASHIMOTO, Satoshi KANECOArticle type: Notes
2017 Volume 33 Issue 10 Pages 1189-1191
Published: October 10, 2017
Released on J-STAGE: October 10, 2017
JOURNAL FREE ACCESSA rapid determination of aniline in environmental water was examined based on liquid chromatography/tandem mass spectrometry (LC/MS/MS). Environmental water samples were diluted 20-fold with Mill-Q water and measured by LC/MS/MS after adding a surrogate substance (aniline-d5). In the results of the present study, the calibration curve of aniline showed good linearity in the range of 0.05 – 2.0 μg/L. Since the RSD (repeatability) by measuring repeatedly an aniline standard solution (0.05 μg/L, n = 7) was 3.2%, the repeatability of this work was very excellent. In addition, the recovery rate of aniline in environmental water was in the range of 99.0 – 102% with RSD 3.4 – 7.7%, and very good recovery test results were obtained. From these results, this analytical method was confirmed to be effective for aniline measurements of environmental water samples. Also, it is possible to conduct rapid analyses of aniline in environmental water without any solid-phase extraction process, compared to the solid-phase extraction-GC/MS method. View full abstractDownload PDF (498K)
View full abstractDownload PDF (498K) -
Yukihiro SHINTANI, Hiroshi KAWARADAArticle type: Notes
2017 Volume 33 Issue 10 Pages 1193-1196
Published: October 10, 2017
Released on J-STAGE: October 10, 2017
JOURNAL FREE ACCESSA polycrystalline diamond electrolyte-solution-gate field-effect transistor (BDD-SGFET) was successfully applied to the analysis of water content in ethanol. Due to the use of a no-gate-insulator FET, the developed sensor showed a four-times-faster response than the conventional Si-FET, and a ten-times-faster response than a glass electrode. The output voltage showed good linearity with respect to the water content. This result is of practical importance because the traditional water content measurement methods are impractical due to their slow response.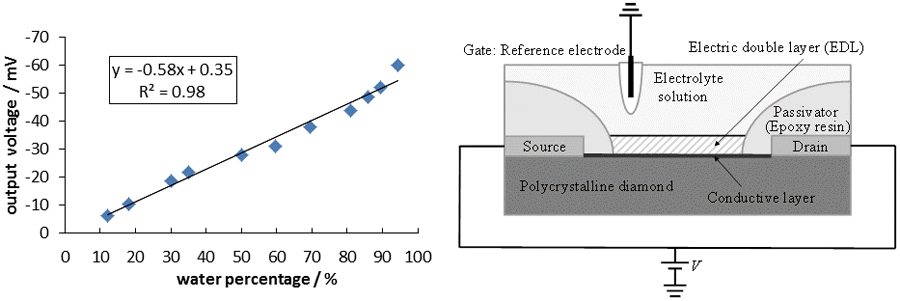 View full abstractDownload PDF (1019K)
View full abstractDownload PDF (1019K) -
Taiga AJIRI, Haruya KASA, Masatoshi MAEKI, Akihiko ISHIDA, Hirofumi TA ...Article type: Notes
2017 Volume 33 Issue 10 Pages 1197-1199
Published: October 10, 2017
Released on J-STAGE: October 10, 2017
JOURNAL FREE ACCESSRecently, we developed a label-free detection method based on optical diffraction, and implemented it in on our fabricated micro- and nanofluidic device. This detection method is simple and useful for detecting biomolecules, but the device fabrication consists of complicated processes. In this paper, we propose a simple method for fabricating the micro- and nanofluidic device; the fabrication combines laser interference lithography with conventional photolithography. The performance of a device fabricated by the proposed method is comparable to the performance of the device in our previous study. View full abstractDownload PDF (1062K)
View full abstractDownload PDF (1062K)
Announcements
-
Article type: Announcements
2017 Volume 33 Issue 10 Pages 1201
Published: October 10, 2017
Released on J-STAGE: October 10, 2017
JOURNAL FREE ACCESSDownload PDF (3164K)
- |<
- <
- 1
- >
- >|