
- |<
- <
- 1
- >
- >|
-
Minkyung Kim, Seoungwoo Shin, Dehun Ryu, Eunae Cho, Jiseon Yoo, Deokho ...2021 Volume 69 Issue 11 Pages 1039-1044
Published: November 01, 2021
Released on J-STAGE: November 01, 2021
Advance online publication: August 28, 2021JOURNAL FREE ACCESS FULL-TEXT HTML
Supplementary materialExposure to UV radiation damages the skin and increases the risk of skin cancer. Sunscreen is used to protect the skin from the harmful effects of UV radiation. However, the chemical UV filters used in sunscreen can show toxicity and cause allergic reactions. A safe sunscreen that includes a lower content of chemical UV filters and exerts an excellent effect on UV protection needs to be developed. The objective of this study was to investigate whether the addition of afzelin to sunscreen could improve the sun protection factor (SPF). A synergistic effect between afzelin and organic sunscreen agents including padimate O and oxybenzone was confirmed. Interestingly, 100% in vitro SPF-boosting was observed when afzelin (0.05%) was applied with a standard SPF formulation containing organic sunscreens while afzelin alone had no contribution to the SPF. In vivo SPF analysis of the standard SPF formulation showed an SPF value of 13.3 that increased to 20.1 when supplemented with afzelin (0.05%). Additionally, afzelin showed no skin irritation in a human trial. These results suggest that afzelin is useful as a natural additive in sunscreen formulations and provides an SPF-boosting effect. Afzelin supplementation to the formulation showed the potential to reduce the use of synthetic photoprotectors, which could minimize the risk of synthetic agent toxicity.
 View full abstractDownload PDF (478K) Full view HTML
View full abstractDownload PDF (478K) Full view HTML -
Shiho Yahata, Mio Hirose, Takayo Ueno, Hiroki Nagumo, Kumiko Sakai-Kat ...2021 Volume 69 Issue 11 Pages 1045-1053
Published: November 01, 2021
Released on J-STAGE: November 01, 2021
JOURNAL FREE ACCESS FULL-TEXT HTML
Supplementary materialFor quantitative analysis, data should be obtained at a sample concentration that is within the range of linearity. We examined the effect of sample concentration on nanoparticle tracking analysis (NTA) of small extracellular vesicles (sEVs), including exosomes, by comparing NTA results of sEVs with those obtained for polystyrene nanoparticles (PSN) and liposomes, which mimic lipid composition and physicochemical properties of exosomes. Initially, NTA of PSN at different concentrations was performed and the particle sizes determined were validated by dynamic light scattering. The major peak maxima for PSN mixtures of different sizes at the higher particle numbers were similar, with some fluctuation of the minor peak maxima observed at the lower particle number, which was also observed for sEVs. Sample concentration is critical for obtaining reproducible data for liposomes and exosomes and increasing the sample concentration caused an increase in data variability because of particle interactions. The inter-day repeatability of particles sizes and concentration for sEVs were 7.47 and 4.51%, respectively. Analysis of the linearity range revealed that this was narrower for sEVs when compared with that of liposomes. Owing to the use of liposomes that mimic the lipid composition and physicochemical properties of exosomes and proteinase-treated sEVs, it was demonstrated that these different analytical results could be possibly caused by the protein corona of sEVs. Consideration of the sample concentration and linearity range is important for obtaining reproducible and reliable data of sEVs.
 View full abstractDownload PDF (1035K) Full view HTML
View full abstractDownload PDF (1035K) Full view HTML -
Yuqin Zhou, Weitong Hu, Xiangli Zhang, Yi Wang, Wenya Zhuang, Fengzhi ...2021 Volume 69 Issue 11 Pages 1054-1060
Published: November 01, 2021
Released on J-STAGE: November 01, 2021
JOURNAL FREE ACCESS FULL-TEXT HTML
Supplementary materialIn the evaluation of the druggability of candidate compounds, it was vital to predict the oral bioavailability of compounds from apparent permeability (Papp) across Caco-2 cell-culture model of intestinal epithelium cultured on commercial transwell plate inserts. The study was to investigate the transport characteristics and permeability of FL118 (10, 11-Methylenedioxy-20(S)-camptothecin) derivatives 7-Q6 (7-(4-Ethylphenyl)-10, 11-methylenedioxy-20(S)-camptothecin) and 7-Q20 (7-(4-Trifluoromethylphenyl)-10, 11-methylenedioxy-20(S)-camptothecin). Transport characteristics and permeability of the tested compounds to the small intestine were assessed at different concentrations (0.5, 1 µM) via Caco-2 cell monolayers model in vitro. Uptake studies based on Caco-2 cells, including temperatures, concentrations, and the influence of efflux transporters, were combined to confirm the transport characteristics of the tested compounds. Furthermore, cytotoxicity results showed that the concentrations used in the experiments were non-toxic and harmless to cells. In addition, The Papp of 7-Q6 was (3.69 ± 1.07) × 10−6 cm/s with efflux ratio (ER) 0.98, while the Papp of 7-Q20 was (7.78 ± 0.89) × 10−6 cm/s with ER 1.05 for apical-to-basolateral (AP→BL) at 0.5 µM, suggesting that 7-Q20 might possess higher oral bioavailability in vivo. Furthermore, P-glycoprotein (P-gp) was proved to slightly affect the accumulations of 7-Q20, while the absorption of 7-Q6 was irrelevant with P-gp and breast cancer resistant protein (BCRP) based on the cellular uptake assays. Accordingly, 7-Q6 was completely absorbed by passive diffusion, and 7-Q20 was mainly dependent on passive diffusion with being effluxed by P-gp slightly. Meanwhile, both 7-Q6 and 7-Q20 were potential antitumor drugs that might exhibit high oral bioavailability in the body.
 View full abstractDownload PDF (869K) Full view HTML
View full abstractDownload PDF (869K) Full view HTML -
Takato Sakurada, Ryo Miyahara, Ryoji Kawazoe, Yusuke Nagata, Yoshiya K ...2021 Volume 69 Issue 11 Pages 1061-1066
Published: November 01, 2021
Released on J-STAGE: November 01, 2021
JOURNAL FREE ACCESS FULL-TEXT HTMLγ-Amido-modified 2′-deoxynucleoside triphosphates (dNTPs) and nucleoside triphosphates (NTPs) are becoming increasingly important as biological tools. We herein describe the simple and easy synthesis of γ-amido-dNTPs and -NTPs from commercially available corresponding dNTPs and NTPs in a one-pot reaction using water-soluble carbodiimide and ammonia solution. We examined the effects of synthesized γ-amido-dNTPs on the DNA polymerase reaction. The results obtained showed the incorporation of these derivatives into the DNA primer while maintaining nucleobase selectivity; however, their incorporation efficiency by DNA polymerase was lower than that of dNTP. This is the first study to demonstrate the successful synthesis of four sets of γ-amido-dNTPs and clarify their properties.
 View full abstractDownload PDF (1352K) Full view HTML
View full abstractDownload PDF (1352K) Full view HTML -
Toshinori Suzuki, Atsuko Ozawa-Tamura, Miyu Takeuchi, Yuriko Sasabe2021 Volume 69 Issue 11 Pages 1067-1074
Published: November 01, 2021
Released on J-STAGE: November 01, 2021
JOURNAL FREE ACCESS FULL-TEXT HTMLDNA reacts directly with UV light with a wavelength shorter than 300 nm. Although ground surface sunlight includes little of this short-wavelength UV light due to its almost complete absorption by the atmosphere, sunlight is the primary cause of skin cancer. Photosensitization by endogenous substances must therefore be involved in skin cancer development mechanisms. Uric acid is the final metabolic product of purines in humans, and is present at relatively high concentrations in cells and fluids. When a neutral mixed solution of 2′-deoxycytidine, 2′-deoxyguanosine, thymidine, and 2′-deoxyadenosine was irradiated with UV light with a wavelength longer than 300 nm in the presence of uric acid, all the nucleosides were consumed in a uric acid dose-dependent manner. These reactions were inhibited by the addition of radical scavengers, ethanol and sodium azide. Two products from 2′-deoxycytidine were isolated and identified as N4-hydroxy-2′-deoxycytidine and N4,5-cyclic amide-2′-deoxycytidine, formed by cycloaddition of an amide group from uric acid. A 15N-labeled uric acid, uric acid-1,3-15N2, having two 14N and two 15N atoms per molecule, produced N4,5-cyclic amide-2′-deoxycytidine containing both 14N and 15N atoms from uric acid-1,3-15N2. Singlet oxygen, hydroxyl radical, peroxynitrous acid, hypochlorous acid, and hypobromous acid generated neither N4-hydroxy-2′-deoxycytidine nor N4,5-cyclic amide-2′-deoxycytidine in the presence of uric acid. These results indicate that uric acid is a photosensitizer for the reaction of nucleosides by UV light with a wavelength longer than 300 nm, and that an unidentified radical derived from uric acid with a delocalized unpaired electron may be generated.
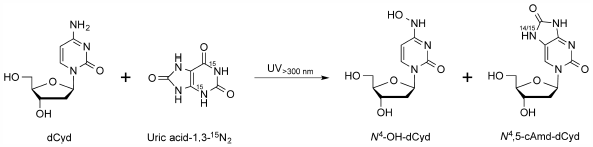 View full abstractDownload PDF (612K) Full view HTML
View full abstractDownload PDF (612K) Full view HTML -
 Kenichi Kawano, Fumiaki Yokoyama, Kouhei Kamasaka, Jun Kawamoto, Takuy ...2021 Volume 69 Issue 11 Pages 1075-1082
Kenichi Kawano, Fumiaki Yokoyama, Kouhei Kamasaka, Jun Kawamoto, Takuy ...2021 Volume 69 Issue 11 Pages 1075-1082
Published: November 01, 2021
Released on J-STAGE: November 01, 2021
JOURNAL FREE ACCESS FULL-TEXT HTML
Supplementary materialExtracellular vesicles (EVs) have emerged as important targets in biological and medical studies because they are involved in diverse human diseases and bacterial pathogenesis. Although antibodies targeting the surface biomarkers are widely used to detect EVs, peptide-based curvature sensors are currently attracting an attention as a novel tool for marker-free EV detection techniques. We have previously created a curvature-sensing peptide, FAAV and applied it to develop a simple and rapid method for detection of bacterial EVs in cultured media. The method utilized the fluorescence/Förster resonance energy transfer (FRET) phenomenon to achieve the high sensitivity to changes in the EV amount. In the present study, to develop a practical and easy-to-use approach that can detect bacterial EVs by peptides alone, we designed novel curvature-sensing peptides, N-terminus-substituted FAAV (nFAAV) peptides. The nFAAV peptides exerted higher α-helix-stabilizing effects than FAAV upon binding to vesicles while maintaining a random coil structure in aqueous solution. One of the nFAAV peptides showed a superior binding affinity for bacterial EVs and detected changes in the EV amount with 5-fold higher sensitivity than FAAV even in the presence of the EV-secretory bacterial cells. We named nFAAV5, which exhibited the high ability to detect bacterial EVs, as an EV-sensing peptide. Our finding is that the coil–α-helix structural transition of the nFAAV peptides serve as a key structural factor for highly sensitive detection of bacterial EVs.
 View full abstractEditor's pick
View full abstractEditor's pickThe authors rationally designed a series of curvature-sensing peptides, which could selectively detect bacterial extracellular vesicles (EVs) in cultured media. The most sensitive peptide nFAAV5, in which the N-terminal region of the previosly reported curvature-sensing peptide FAAV was modified, was applicable for quantification of changes in the amount of EVs even in the presence of the EV-secretory bacterial cells. Therefore, the novel peptide is useful to EV studies as a potent EV-sensing peptide.
Download PDF (2042K) Full view HTML -
Kaname Hashizaki, Yuto Hoshii, Kosuke Ikeuchi, Miko Imai, Hiroyuki Tag ...2021 Volume 69 Issue 11 Pages 1083-1087
Published: November 01, 2021
Released on J-STAGE: November 01, 2021
JOURNAL FREE ACCESS FULL-TEXT HTMLOur aim was to determine the surface free energy (SFE) of semi-solid dosage forms (SSDFs) by establishing a reproducible method for measuring the contact angle of liquids to SSDFs. Four SSDFs were used: petrolatum, an oil/water (O/W) and a water/oil (W/O) cream, and an alcohol-based gel. The SSDFs were evenly spread on a glass slide, and the change in contact angle over time was measured by dropping water, glycerol, diiodomethane and n-hexadecane as the test liquids. Depending on the combination of test liquid and SSDF, the contact angle was either constant or decreased in an exponential manner. Contact angles may have decreased in an exponential manner because the reaction between the test liquid and the SSDF altered the interfacial tension between the two phases and changed the surface tension of the test liquid and the SFE of the SSDF. The contact angle of the test liquid to the SSDF could be determined reproducibly using the initial contact angle immediately after dropping the liquid on the SSDF as the contact angle before reaction. Using the obtained contact angles and the Owens–Wendt–Rabel–Kaelble equation, we calculated the SFE and its component for the SSDFs tested and found that the results reflect the physicochemical properties of SSDFs. Furthermore, the work of adhesion (WA) of the SSDF to Yucatan micropig skin was calculated using the SFE for the SSDFs. Interestingly, the WA values for all SSDFs tested were comparable.
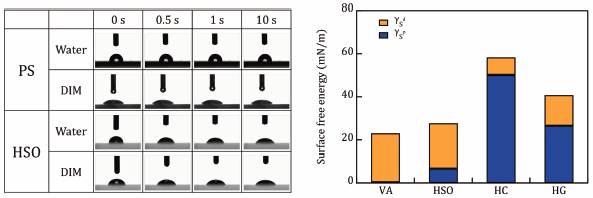 View full abstractDownload PDF (704K) Full view HTML
View full abstractDownload PDF (704K) Full view HTML -
 Hiroki Katayama, Yoshiharu Maeda, Tsubasa Sato, Asami Mogi, Shoko Itak ...2021 Volume 69 Issue 11 Pages 1088-1096
Hiroki Katayama, Yoshiharu Maeda, Tsubasa Sato, Asami Mogi, Shoko Itak ...2021 Volume 69 Issue 11 Pages 1088-1096
Published: November 01, 2021
Released on J-STAGE: November 01, 2021
JOURNAL FREE ACCESS FULL-TEXT HTMLFrom the viewpoint of self-medication, it is valuable to develop patient-friendly scored tablets that possess dividing uniformity. In this context, we attempted to optimize the preparation conditions for a tablet with a unique shape, such as a concavely curved scored tablet (CCST). Employing a design of experiment and a response surface method incorporating a thin-plate spline interpolation, and a bootstrap resampling technique, the optimal preparation conditions for CCST were successfully developed. To make it possible to scaleup the optimal solution estimated on a trial-scale, a Bayesian estimation was applied. Credible ranges of critical responses in large-scale manufacturing were estimated as a posterior probability from the trial-scale experiment as a prior probability. In terms of the large-scale manufacturing, the possibility of solving the scaleup problem was suggested using Bayesian estimation. Furthermore, a simulation study using a finite element method revealed that strong tensile stresses generated along the tip of the score line in CCST when an outer force was applied to the back surface of CCST. An advantage in dividing uniformity is indicated by the unique shape of CCST.
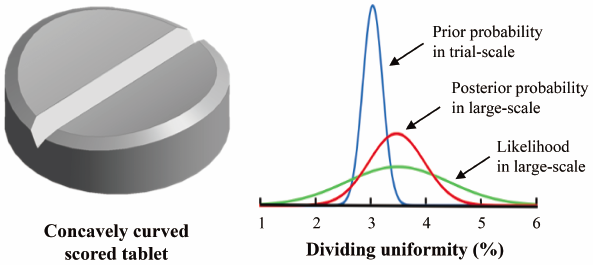 View full abstractEditor's pick
View full abstractEditor's pickIn view of self-medication, it is valuable to develop patient-friendly scored tablets that possess dividing uniformity. Preparation conditions for a concavely curved scored tablet were optimized, employing a design of experiment and a response surface method incorporating a thin-plate spline interpolation, and a bootstrap resampling technique. To make it possible to scaleup the optimal solution estimated on a trial-scale, a Bayesian estimation was applied. Credible ranges of critical responses in large-scale manufacturing were successfully estimated as a posterior probability from the trial-scale experiment as a prior probability.
Download PDF (6066K) Full view HTML -
Akiko Asano, Chisato Minami, Shiori Matsuoka, Takuma Kato, Mitsunobu D ...2021 Volume 69 Issue 11 Pages 1097-1103
Published: November 01, 2021
Released on J-STAGE: November 01, 2021
JOURNAL FREE ACCESS FULL-TEXT HTML
Supplementary materialThe structure of an ornithine (Orn)-free Gramicidin S (GS) analogue, cyclo(Val–Nle–Leu–D-Phe–Pro)2 (NGS), was studied. Its circular dichroism (CD) spectrum showed that NGS has a structure similar to GS, though the value of [θ] indicated smaller β-turn and sheet populations. This is probably because the Nle side chain could not form intramolecular hydrogen bonds stabilizing the sheet structure. The chemical shift perturbation of αH and JNH–αH were similar in GS and NGS. Three independent NGS molecules formed intramolecular β-sheet structures in crystal. The turn structures of D-Phe-Pro moieties were classed as type II′ β-turns, but one part was unclassed. The molecules were arranged in a twisting manner, which resulted in the formation of a helical sheet. Similar structural characteristics were observed previously in a Leu-type, Orn-free GS analogue and in GS trifluoroacetic acid salt.
 View full abstractDownload PDF (2472K) Full view HTML
View full abstractDownload PDF (2472K) Full view HTML -
 Huihui Zhang, Rihui Cao, Feng Zeng, Wenxi Fan, Liang Guo, Qin Ma, Shao ...2021 Volume 69 Issue 11 Pages 1104-1109
Huihui Zhang, Rihui Cao, Feng Zeng, Wenxi Fan, Liang Guo, Qin Ma, Shao ...2021 Volume 69 Issue 11 Pages 1104-1109
Published: November 01, 2021
Released on J-STAGE: November 01, 2021
JOURNAL FREE ACCESS FULL-TEXT HTMLIn this study, a series of alkyl diamine linked bivalent β-carbolines was synthesized and evaluated as antitumor agent. The results demonstrated that most compounds displayed good antiproliferative activities with IC50 value lower than 10 µM against a panel of human tumor cell lines, and compound 8 was found to be the most potent antiproliferative agent with IC50 value of 1.39, 1.96, 1.42, 1.49, 1.32, 1.96 and 1.63 µM against human breast cancer cell line (MCF-7), human adenocarcinoma cell line (769-P), human malighant melanoma cell line (A375), human ovarian cancer cell line (SK-OV-3), human colon carcinoma cell line (HCT-116), human gastric cancer cell line (BGC-823) and human esophageal squamous carcinoma cell line (Eca-109), respectively. Further investigations on mechanism of action of this class of compound demonstrated that the representative compound 8 inhibited colorectal cancer growth through inducing autophagy.
 View full abstractEditor's pick
View full abstractEditor's pickAutophagy plays diverse functional roles in various stages of tumor, and numerous drugs targeting autophagy in in-vitro and in-vivo models for colorectal cancer (CRC) have been reported. In search for novel and effective drugs with minimal cytotoxicity to reduce the mortality rate of CRC, the authors designed and synthesized a series of alkyl diamine linked bivalent β-carbolines, and compound 8 was found to be the most potent antitumor agent against human colon carcinoma cell lines with the IC50 value of less than 5 μM. The mechanism of action study revealed that compound 8 reduced growth of colon cancer cells by inducing autophagy.
Download PDF (1091K) Full view HTML -
 Mayuko Akiu, Takashi Tsuji, Kouki Iida, Yoshitaka Sogawa, Koji Terayam ...2021 Volume 69 Issue 11 Pages 1110-1122
Mayuko Akiu, Takashi Tsuji, Kouki Iida, Yoshitaka Sogawa, Koji Terayam ...2021 Volume 69 Issue 11 Pages 1110-1122
Published: November 01, 2021
Released on J-STAGE: November 01, 2021
JOURNAL FREE ACCESS FULL-TEXT HTMLNicotinamide phosphoribosyltransferase (NAMPT) catalyzes the rate-limiting step of the nicotinamide adenine dinucleotide (NAD+) salvage pathway. Because NAD+ plays a pivotal role in energy metabolism and boosting NAD+ has positive effects on metabolic regulation, activation of NAMPT is an attractive therapeutic approach for the treatment of various diseases, including type 2 diabetes and obesity. Herein we report the discovery of 1-(2-phenyl-1,3-benzoxazol-6-yl)-3-(pyridin-4-ylmethyl)urea 12c (DS68702229), which was identified as a potent NAMPT activator. Compound 12c activated NAMPT, increased cellular NAD+ levels, and exhibited an excellent pharmacokinetic profile in mice after oral administration. Oral administration of compound 12c to high-fat diet-induced obese mice decreased body weight. These observations indicate that compound 12c is a promising anti-obesity drug candidate.
 View full abstractEditor's pick
View full abstractEditor's pickNicotinamide phosphoribosyltransferase (NAMPT) catalyzes the rate-limiting step of the NAD+ salvage pathway. Since boosting NAD+ has positive effects on metabolic regulation, activation of NAMPT is an attractive therapeutic approach for the treatment of various diseases, including type 2 diabetes and obesity. Optimization of our previous lead compound led to identification of DS68702229, a potent NAMPT activator with good oral exposure. Oral administration of DS68702229 to high-fat diet-induced obese mice elicited NAD+ level increase in various tissues, ultimately leading to continuous and significant body weight reduction. These observations indicate that DS68702229 is a promising anti-obesity drug candidate.
Download PDF (1171K) Full view HTML -
Hiroshi Tateishi, Mika Tateishi, Mohamed O Radwan, Takuya Masunaga, Ko ...2021 Volume 69 Issue 11 Pages 1123-1130
Published: November 01, 2021
Released on J-STAGE: November 01, 2021
JOURNAL FREE ACCESS FULL-TEXT HTML
Supplementary materialA disintegrin and metalloproteinase 17 (ADAM17) is a zinc-dependent enzyme that catalyzes the cleavage of the extracellular domains of various transmembrane proteins. ADAM17 is regarded as a promising drug target for the suppression of various diseases, including cancer metastasis. We synthesized a new ADAM17 inhibitor, SN-4, composed of a zinc-binding dithiol moiety and an appendage that specifically binds to a pocket of ADAM17. We show that SN-4 inhibits the ability of ADAM17 to cleave tumor necrosis factor α (TNF-α) in vitro. This activity was reduced by the addition of zinc, indicating the importance of the zinc chelating dithiol moiety. Inhibition of TNF-α cleavage by SN-4 in cells was also observed, and with an IC50 of 3.22 µM, SN-4 showed slightly higher activity than the well-studied ADAM17 inhibitor marimastat. Furthermore, SN-4 was shown to inhibit cleavage of CD44 by ADAM17, but not by ADAM10, and to suppress cell invasion. Molecular docking showed good fitting of the specificity pocket-binding group and one SH of SN-4 and hinted at possible means of structural optimization. This study provides clues for the development of potent and selective ADAM17 inhibitors.
 View full abstractDownload PDF (2712K) Full view HTML
View full abstractDownload PDF (2712K) Full view HTML
-
Shinya Kimura, Nana Haraya, Tomoki Komiyama, Masashi Yokoya, Masamichi ...2021 Volume 69 Issue 11 Pages 1131-1135
Published: November 01, 2021
Released on J-STAGE: November 01, 2021
JOURNAL FREE ACCESS FULL-TEXT HTMLAn amphiphilic tris-urea compound (1) containing hydrophilic resorcinol units was designed and synthesized. Compound 1 formed supramolecular hydrogels in basic buffers, such as glycine–NaOH, phosphate–NaOH, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES)–NaOH, and borate–NaOH. The optimum pH range of the buffer solution for gelation was 10–11 and insoluble suspensions or solutions were formed when the pH was outside this range. When the borate–NaOH buffer was used, supramolecular hydrogels were formed over a wide pH range (7.5–11.0). The thermal stabilities and viscoelastic properties of the supramolecular hydrogels were determined from the gel-to-sol phase transition temperatures and rheological properties, respectively. The supramolecular hydrogel formed from compound 1 and the borate–NaOH buffer exhibited a pH-responsive reversible gel-to-sol phase transition property. Gel-to-sol phase transition could be achieved by adding NaOH and regelation of the sol was realized by adding an appropriate amount of boric acid. Increasing the amount of the acid resulted in a gel-to-sol phase transition.
 View full abstractDownload PDF (1310K) Full view HTML
View full abstractDownload PDF (1310K) Full view HTML -
Tran Thi Minh, Ho Khanh Toan, Hoang Thi Lan Anh, Tran Thu Huong, Do Th ...2021 Volume 69 Issue 11 Pages 1136-1139
Published: November 01, 2021
Released on J-STAGE: November 01, 2021
JOURNAL FREE ACCESS FULL-TEXT HTML
Supplementary materialA phytochemical investigation of methanol extract from leaves of Pachyrhizus erosus (L.) Urban, a leguminous shrub distributed in Vietnam and other tropical and subtropical countries led to the isolation of a new prenylated chalcone, erosusone (1) and a new megastigmane glycoside epimer, 3-episedumoside F1 (9), together with thirteen known compounds including flavonoids (2–6), a 3-benzoxepine lactone (7), a pyridine-4,5-diol derivative (8), megastigmanes and megastigmane glycosides (10–15). Their structures were elucidated by means of high resolution-electrospray ionization (HR-ESI)-MS, one dimensional (1D) and two-dimensional NMR (2D-NMR) spectroscopy as well as comparison with the data reported in the literature. The cytotoxic effects on LU-1 (lung carcinoma), HepG2 (hepatocellular carcinoma), and MCF7 (breast carcinoma) cell lines were assessed. Prenylated chalcones 1–2 and isoflavone 3 exhibited cytotoxicity against all tested cell lines with IC50 values ranging from 22.04 to 45.03 µM.
 View full abstractDownload PDF (499K) Full view HTML
View full abstractDownload PDF (499K) Full view HTML
-
2021 Volume 69 Issue 11 Pages 1140
Published: November 01, 2021
Released on J-STAGE: November 01, 2021
JOURNAL FREE ACCESS FULL-TEXT HTMLDownload PDF (143K) Full view HTML
- |<
- <
- 1
- >
- >|