
- |<
- <
- 1
- >
- >|
-
Hayato Ishikawa2025Volume 73Issue 2 Pages 67-77
Published: February 01, 2025
Released on J-STAGE: February 01, 2025
JOURNAL OPEN ACCESS FULL-TEXT HTMLDespite the great strides in biopharmaceuticals and monoclonal antibodies today, natural products remain highly attractive as drug candidates. Therefore, building a library of natural products through total synthesis is critically important for drug discovery. This perspective article details the collective total synthesis of polycyclic natural products using “bioinspired reactions” that mimic natural product biosynthesis. It discusses the total syntheses of 20 natural products, including dimeric diketopiperazine alkaloids, monoterpenoid indole alkaloids, and iridoid glycosides, each achieved in fewer than 14 steps starting from commercially available materials.
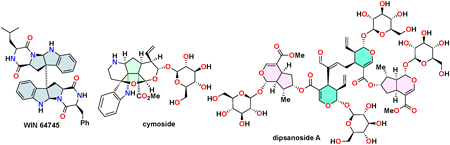 View full abstractDownload PDF (770K) Full view HTML
View full abstractDownload PDF (770K) Full view HTML
-
Kenta Fujinuma, Masataka Ito, Etsuo Yonemochi, Katsuhide Terada, Hiron ...2025Volume 73Issue 2 Pages 78-85
Published: February 04, 2025
Released on J-STAGE: February 04, 2025
JOURNAL OPEN ACCESS FULL-TEXT HTML
Supplementary materialMagnesium stearate (MgSt), which is often used as a lubricant in the production of solid formulations, has different stearic acid and palmitic acid contents depending on the lot and manufacturer, resulting in differences in mixability and lubricating effect. However, there are few reports on the effect of the different physical properties of MgSt samples on mixing and tablet formation. Additionally, it is known that the triboelectric properties of components affect the mixability. Overmixing of formulations can decrease tablet hardness. In this study, the triboelectric properties and mixability of MgSt samples with different lots and manufacturers were evaluated. Tablet hardness was used as an index of the mixability of the MgSt samples with various excipients. The triboelectric properties of the MgSt samples depended on the production lot. Mixability was higher when the triboelectric properties of the excipient and MgSt were different. By evaluating the charge properties of MgSt, it should be possible to select the optimum lot and manufacturer of MgSt for specific formulations.
 View full abstractDownload PDF (3747K) Full view HTML
View full abstractDownload PDF (3747K) Full view HTML -
 Kei Kitamura, Taiki Yamasaki, Hiroto Kaku2025Volume 73Issue 2 Pages 86-93
Kei Kitamura, Taiki Yamasaki, Hiroto Kaku2025Volume 73Issue 2 Pages 86-93
Published: February 06, 2025
Released on J-STAGE: February 06, 2025
JOURNAL OPEN ACCESS FULL-TEXT HTML
Supplementary materialPrealnumycin (1), a benzoisochromanequinone compound, produces biologically active exfoliamycin or alnumycin through hybridization with D-ribose or oxidation. We report herein a concise and stereoselective synthesis of 1. The anionic annulation of phthalide 5 with enone 6, prepared via a transition metal-catalyzed enantioselective route, afforded tricyclic lactone 4. This intermediate then underwent a highly diastereoselective introduction of an n-propyl group through nucleophilic addition followed by silane reduction. Subsequent regioselective arene oxidation of 18 using cerium(IV) ammonium nitrate (CAN) afforded naphthoquinone 2. Further manipulations, including acidic deprotection and elimination, yielded prealnumycin in 8 steps.
 View full abstractEditor's pick
View full abstractEditor's pickThe alnumycin-class antibiotics constitute a polyketide-derived benzoisochromanequinone core hybridized with a structurally rearranged D-ribose. In this article, the authors reported the stereoselective synthesis and absolute configuration of prealnumycin, the aglycon of alnumycin. The key transformation involves the highly diastereoselective introduction of an n-propyl group onto a tricyclic lactone via nucleophilic addition, followed by silane reduction. Subsequent regioselective arene oxidation to naphthoquinone, acidic deprotection, and dehydration afford prealnumycin in eight steps. The findings from this synthesis provide insights into the total synthesis of this class of natural products.
Download PDF (997K) Full view HTML -
 Norikazu Miyamoto, Kenta Ohsugi, Taishi Higashi, Keiichi Motoyama2025Volume 73Issue 2 Pages 94-102
Norikazu Miyamoto, Kenta Ohsugi, Taishi Higashi, Keiichi Motoyama2025Volume 73Issue 2 Pages 94-102
Published: February 11, 2025
Released on J-STAGE: February 11, 2025
JOURNAL OPEN ACCESS FULL-TEXT HTML
Supplementary materialThe pharmaceutical industry relies heavily on the safe and efficient packaging of drugs and injection glass vials play a pivotal role in this regard. Ensuring the quality and consistency of these vials is essential for safeguarding the potency of pharmaceutical formulations. In this study, the recent breakthroughs achieved in the manufacturing of injection glass vials by implementing advanced surface-processing technologies were examined. We developed potential injection glass vials using the novel vial-inner-surface treatment (VIST) technology to homogenize the inner surface of the vials. Compared with common vials, the elution of alkali contents and conductivity of these injection glass vials were reduced because of the VIST technology, resulting in the formation of smooth and homogeneous inner surfaces. In addition, drug adsorption onto the inner surface of the VIST vials was considerably lowered than that onto common vials. These results suggest that VIST vials are of excellent quality and could become the standard injection glass vials.
 View full abstractEditor's pick
View full abstractEditor's pickThe authors developed potential injection glass vials by using the novel vial-inner-surface treatment (VIST) technology to homogenize the inner surface of the vials. Compared with those of common vials, the elution of alkali contents and conductivity of these injection glass vials were reduced because of the VIST technology resulting in the formation of smooth and homogeneous inner surfaces of the vials. In addition, drug adsorption onto the inner surface of the VIST vials was considerably lowered than that onto common vials. These results suggest that VIST vials are of excellent quality and could become the standard injection glass vials.
Download PDF (9184K) Full view HTML
-
 Yoshimi Ichimaru, Koichi Kato, Yoshihiro Yamaguchi, Takayuki Sakamoto, ...2025Volume 73Issue 2 Pages 103-107
Yoshimi Ichimaru, Koichi Kato, Yoshihiro Yamaguchi, Takayuki Sakamoto, ...2025Volume 73Issue 2 Pages 103-107
Published: February 15, 2025
Released on J-STAGE: February 15, 2025
JOURNAL OPEN ACCESS FULL-TEXT HTML
Supplementary materialHere, a DNA cleavage reagent (1-(anthracen-9-ylmethyl)-1,5,9-triazacyclododecane = Ant-[12]aneN3) was designed and synthesized, and its DNA photocleavage activity under UV irradiation at λ = 365 nm was evaluated. Ant-[12]aneN3 is a molecule containing anthracene as the photosensitizer and [12]aneN3 ( = 1,5,9-triazacyclododecane) as the DNA-interacting component. The cyclic polyamine [12]aneN3 could coordinate with zinc ions (ZnII) and affect DNA cleavage activity. Therefore, when Ant-[12]aneN3 reacted with Zn(NO3)‧6H2O, the product was not a ZnII complex but an N-protonated form of Ant-[12]aneN3. In DNA cleavage experiments with the pUC19 plasmid, Ant-[12]aneN3 also showed DNA photocleavage activity in a ZnII-independent manner. That is, [12]aneN3 enhances the DNA photocleavage activity of anthracene in a ZnII-independent manner, unlike bpa (bis(2-picolyl)amine), which was previously reported to enhance DNA cleavage activity by chelating ZnII. Under physiological conditions, the nitrogen atoms of [12]aneN3 appear protonated without the addition of ZnII salts and showed an affinity for the negatively charged DNA. The results of this study may facilitate the design of effective DNA cleavage reagents.
 View full abstractEditor's pick
View full abstractEditor's pickIn this manuscript, the authors described the design and synthesis of a derivative of 1,5,9-triazacyclododecane ([12]aneN3) bearing an anthracen-9-ylmethyl moiety (Ant-[12]aneN3) and evaluated its DNA cleavage activity under UV irradiation at 365 nm. They found that its DNA cleavage activity was dependent on UV irradiation but not on the presence of zinc ions, despite the expectation that the [12]aneN3 moiety would facilitate DNA cleavage through zinc ion chelation. The authors also investigated the DNA cleavage activity of Ant-[12]aneN3 in comparison to structurally related compounds and elucidated the unique role of the [12]aneN3 moiety in Ant-[12]aneN3 in DNA cleavage. This work would contribute to the field of cyclic polyamines and the photoreactivity of the anthracene moiety.
Download PDF (1759K) Full view HTML -
Mizuho Yamasaki, Masayuki Munekane, Kento Kannaka, Kohei Sano, Toshihi ...2025Volume 73Issue 2 Pages 108-111
Published: February 21, 2025
Released on J-STAGE: February 21, 2025
JOURNAL OPEN ACCESS FULL-TEXT HTML
Supplementary materialWe developed a novel drug release method using a bioorthogonal inverse electron demand Diels–Alder reaction on liposomal membranes. Based on reports that replacing pyridine with pyrimidine in tetrazine derivatives improves the reaction rate with strained dienophiles, we investigated if liposomes with tetrazine derivatives containing pyrimidine rings efficiently release drugs via click chemistry. We synthesized and evaluated a tetrazine compound (Tz2) bearing a pyrimidine ring. The reaction rate constant of Tz2 with a norbornene (NB) derivative, 5-norbornenecarboxylic acid (NBCOOH), was higher than that of Tz1 with a pyridine ring. Liposomes containing the synthesized Tz2 (Tz2-liposomes) were prepared, and the reaction between Tz2 and NBCOOH on the liposomal membranes was confirmed using high-resolution mass spectrometry. We encapsulated indium-111-labeled diethylenetriaminepentaacetic acid ([111In]In-DTPA) in liposomes as a model drug. The release of [111In]In-DTPA from Tz2-liposomes was observed after the addition of NBCOOH, with release dependent on NBCOOH concentration. Moreover, release from Tz2-liposomes was significantly higher than that from Tz1-liposomes. These results suggested that tetrazine derivatives with pyrimidine rings efficiently released drugs, likely due to enhanced reaction rates. These findings would advance the development of controlled drug release methods using click chemistry.
 View full abstractDownload PDF (1084K) Full view HTML
View full abstractDownload PDF (1084K) Full view HTML
-
Yaqi Meng, Yuqing Wang, Shujiao Li, Zhiyan Cai, Guo Zhuang, Yanli Yang2025Volume 73Issue 2 Pages 112-120
Published: February 26, 2025
Released on J-STAGE: February 26, 2025
JOURNAL OPEN ACCESS FULL-TEXT HTML
Supplementary materialEupatilin, a natural bioactive flavone, is the active ingredient in traditional Chinese medicine Artemisia argyi Levl. et Vant. To enhance the antitumor effect of eupatilin, we designed a series of novel eupatilin–Mannich derivatives and investigated antitumor activity against several human cancer cell lines, including gastric cancer cells (AGS), esophageal cancer cells (Eca-109), and breast cancer cells (MDA-MB-231). Among all derivatives, the majority demonstrated superior antitumor activity compared to eupatilin, with compound 3d exhibiting the most effective antitumor activity against AGS cells. Furthermore, compound 3d effectively inhibited colony formation and migration of AGS cells. Network pharmacology combined with molecular docking studies indicated that compound 3d exerts antitumor activity by targeting the Hsp90AA1 and multiple signaling pathways. In addition, the Western blot experiment results showed that compound 3d reduced the expression of Hsp90AA1 in AGS cells, indicating that Hsp90AA1 may be the potential target of compound 3d. In summary, several novel eupatilin derivatives were prepared via the Mannich reaction, representing the first structure modification study of eupatilin. The mechanism of action of compound 3d was estimated through cell experiments, network pharmacology, molecular docking, and Western blot experiments, to provide lead compounds for the discovery of natural product-based antitumor candidates.
 View full abstractDownload PDF (7777K) Full view HTML
View full abstractDownload PDF (7777K) Full view HTML -
 Ion-Pair Extraction with Tetracyanocyclopentadienides: A Method for Estimating Extraction EfficiencyTakeo Sakai, Masanari Ota, Miho Ito, Riho Miura, Yuto Fumimoto, Yuji M ...2025Volume 73Issue 2 Pages 121-135
Ion-Pair Extraction with Tetracyanocyclopentadienides: A Method for Estimating Extraction EfficiencyTakeo Sakai, Masanari Ota, Miho Ito, Riho Miura, Yuto Fumimoto, Yuji M ...2025Volume 73Issue 2 Pages 121-135
Published: February 28, 2025
Released on J-STAGE: February 28, 2025
JOURNAL OPEN ACCESS FULL-TEXT HTML
Supplementary materialIon-pair extraction with tetracyanocyclopentadienides (TCCPs) is an effective method for isolating ammonium cations; however, predicting the extractability of ammonium-TCCP ion pairs is challenging. Herein, the measured extraction coefficients (LogKex) of ammonium-TCCP ion pairs allowed the values of A (an index for anion extractability) for the TCCP anions to be determined (–3.1 to –1.4); these values were higher than and similar to those of perchlorate and picrate anions, respectively. The correlation between LogKex and the sum of the values of A and the calculated Log P of ammonium cations revealed that LogKex can be estimated by the equation LogKex = A + CLOGPNR4 + 1.6, where CLOGPNR4 is the CLOGP value obtained using the “no-plus” ammonium structure. The LogKex value can be used to predict the extractability of quaternary ammonium ions.
 View full abstractEditor's pick
View full abstractEditor's pick[Highlighted Paper selected by Editor-in-Chief]
Quaternary ammonium cations are difficult to handle in organic chemistry owing to their ionic nature. Ion-pair extraction is commonly adopted for isolating quaternary ammonium cations; however, predictions of extraction efficiency are often based on researchers’ intuition. In this study, the authors derived an equation to predict the extraction efficiency of ammonium–tetracyanocyclopentadienide ion pairs. The prediction equation involves the CLOGP values of ammonium and lipophilic constants of anions and is also applicable to ion-pair extraction with many common anions. These findings will aid the development of future studies using ammonium cations as synthetic substrates and products.Download PDF (3760K) Full view HTML
- |<
- <
- 1
- >
- >|