
- |<
- <
- 1
- >
- >|
-
Takayuki Ichikawa, Hiroki Miyaoka, Makoto Hino, Keitaro Horikawa, Teru ...Article type: Regular Article
2020 Volume 84 Issue 3 Pages 67
Published: March 01, 2020
Released on J-STAGE: February 25, 2020
JOURNAL FREE ACCESS FULL-TEXT HTML -
Keitaro Horikawa, Hidetoshi KobayashiArticle type: Regular Article
2020 Volume 84 Issue 3 Pages 68-73
Published: March 01, 2020
Released on J-STAGE: February 25, 2020
Advance online publication: October 21, 2019JOURNAL FREE ACCESS FULL-TEXT HTMLIn the present study, a new method for hydrogen charging in aluminum was developed which named friction in water (FW) process. The hydrogen charging was carried out by polishing aluminum surfaces for 1 h with a silicon carbide emery paper in water at room temperature. For comparison, hydrogen-uncharged specimens were also prepared. The total amount of hydrogen in aluminum clearly increased after the FW process, during which hydrogen was generated by the chemical reaction between water and the non-oxide coated aluminum. After hydrogen charging by the FW process, aluminum showed a decrease in ductility in conventional strain rate tensile testing. Hydrogen microprint technique (HMT) revealed that hydrogen atoms absorbed by the FW process was diffused to the opposite surface in the thickness direction.
 Fig. 3 Schematic of the connection of sensor gas chromatography and the reaction container. Fullsize ImageView full abstractDownload PDF (3715K) Full view HTML
Fig. 3 Schematic of the connection of sensor gas chromatography and the reaction container. Fullsize ImageView full abstractDownload PDF (3715K) Full view HTML -
Ryota Kido, Ryoichi Kuwano, Makoto Hino, Keisuke Murayama, Seigo Kuros ...Article type: Regular Article
2020 Volume 84 Issue 3 Pages 74-79
Published: March 01, 2020
Released on J-STAGE: February 25, 2020
Advance online publication: January 27, 2020JOURNAL FREE ACCESS FULL-TEXT HTMLIn this study, the effect of anodization and electroless Ni-P plating on the fatigue strength of commercial A5052-H14 and A2017-T4 aluminum alloys was investigated. The coated aluminum alloys were tested using a rotary bending fatigue testing machine. Anodization led to a slight increase in the fatigue strength of the A2017-T4 alloy of approximately 10% because of the suppression of the generation of fatigue crack, and anodization with a 5-µm thickness for A5052-H14 also led to a slight increase in the fatigue strength. However, anodization with a 20-µm thickness for A5052-H14 led to reduced fatigue strength because of the pits that formed in the film. In addition, electroless Ni-P plating drastically improved the fatigue strength of the A5052-H14 alloy by suppressing the generation of fatigue crack.It also improved the fatigue strength of the A2017-T4 alloy in the high-stress region. However, the fatigue strength in the low-stress region was the same as that of the non-coated specimens.This fatigue strength should have originated from the hydrogen embrittlement by the hydrogen introduced into the specimen during the plating.
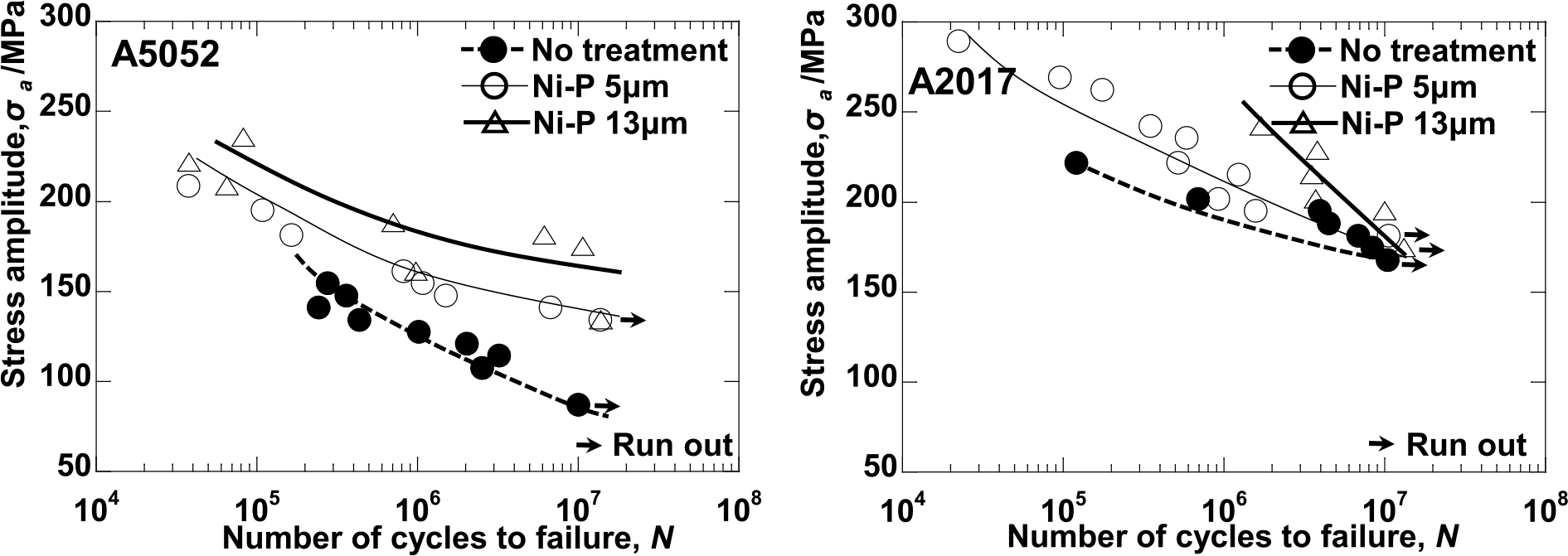 Fig. 7 Relation between stress (σ) and number of cycles to failure (N) for electroless Ni-P plated specimens. Fullsize ImageView full abstractDownload PDF (2858K) Full view HTML
Fig. 7 Relation between stress (σ) and number of cycles to failure (N) for electroless Ni-P plated specimens. Fullsize ImageView full abstractDownload PDF (2858K) Full view HTML -
Makoto Hino, Yuho Doi, Ryoichi Kuwano, Yukinori Oda, Keitaro HorikawaArticle type: Regular Article
2020 Volume 84 Issue 3 Pages 80-86
Published: March 01, 2020
Released on J-STAGE: February 25, 2020
Advance online publication: January 27, 2020JOURNAL FREE ACCESS FULL-TEXT HTMLHydrogen embrittlement of SK85 high-strength steel sheets was evaluated through a three-point bending test. The effects of electroless Ni-P plating and Ni electroplating on hydrogen embrittlement were examined with respect to the hydrogen permeability of the plated films. On the morrow of the plating, electroless Ni-P plating indicates a high degree of hydrogen embrittlement, irrespective of the phosphorus content in the film. However, hydrogen embrittlement of Ni electroplating is further suppressed than that of electroless Ni-P plating, which can be attributed to the excellent hydrogen diffusing ability of Ni electroplating. These results demonstrate that the hydrogen permeability of a plated film is an important factor for hydrogen embrittlement. Hydrogen present on the plated film surface was visualized by employing the hydrogen microprint technique, and the corresponding results reveal that the hydrogen permeability of the plated film is dependent on the film crystal structure. Furthermore, the pits in the film can become a route for hydrogen emission.
 Fig. 5 Relationships between the time left standing from the plating and the hydrogen embrittlement rate. (a) Electroless Ni-P, Low P (b) Electroless Ni-P, Middle P (c) Electroless Ni-P, High P (d) Ni electroplating. Fullsize ImageView full abstractDownload PDF (2040K) Full view HTML
Fig. 5 Relationships between the time left standing from the plating and the hydrogen embrittlement rate. (a) Electroless Ni-P, Low P (b) Electroless Ni-P, Middle P (c) Electroless Ni-P, High P (d) Ni electroplating. Fullsize ImageView full abstractDownload PDF (2040K) Full view HTML -
Makoto Hino, Shunsuke Mukai, Takehiro Shimada, Koki Okada, Keitaro Hor ...Article type: Regular Article
2020 Volume 84 Issue 3 Pages 87-91
Published: March 01, 2020
Released on J-STAGE: February 25, 2020
Advance online publication: January 27, 2020JOURNAL FREE ACCESS FULL-TEXT HTMLThe hydrogen embrittlement of SK85 high-strength steel sheets was evaluated using a three-point bending test. The effects of electroplating each with zinc, zinc-SiO2, zinc-nickel, and zinc-nickel-SiO2 on hydrogen embrittlement were examined by baking the electroplated steel specimen. Each electroplating type caused hydrogen embrittlement, which was promoted by hydrogen, owing to the reduction due to hydrogen ions during electroplating. The hydrogen embrittlement of both zinc-electroplated and zinc-SiO2-electroplated SK85 steel continued after baking for 24 h at 200℃, but that of the zinc-nickel-electroplated and zinc-nickel-SiO2-electroplated SK85 steel ceased. Furthermore, TDA revealed that the diffusible hydrogen at approximately 200℃, which was caused because of hydrogen embrittlement, was desorbed from all the electroplated specimens before the baking. However, after the baking, this diffusible hydrogen for each specimen was not desorbed. These results indicate that the hydrogen embrittlement for zinc-based electroplated high-strength steel was caused by another factor except for diffusible hydrogen. The hydrogen formed due to the electroplating was incorporated in the steel substrate, following which the hydrogen-vacancy cluster was formed in the substrate. It seems that the zinc and zinc-SiO2 film provided insufficient permeability required for the formation of the hydrogen-vacancy cluster. However, zinc–nickel and zinc-nickel-SiO2 film enabled hydrogen-vacancy cluster diffusion from the substrate.
 Fig. 2 Relationship between the hydrogen embrittlement rate and the baking time for various electroplating. Fullsize ImageView full abstractDownload PDF (1426K) Full view HTML
Fig. 2 Relationship between the hydrogen embrittlement rate and the baking time for various electroplating. Fullsize ImageView full abstractDownload PDF (1426K) Full view HTML -
Ryo Kawakami, Kazumasa Kubota, Hisao MatsunagaArticle type: Regular Article
2020 Volume 84 Issue 3 Pages 92-98
Published: March 01, 2020
Released on J-STAGE: February 25, 2020
JOURNAL FREE ACCESS FULL-TEXT HTMLThe method of rotating bending fatigue test under an electrochemical hydrogen-charging was established, by which the fatigue tests were carried out for a JIS-SCM435 to study the effect of hydrogen on fatigue strength. The fatigue life of the specimen was degraded by hydrogen-charging in relatively low fatigue life regime, where the slope of S-N curve for the hydrogen-charged specimens was equivalent to that for no-charged specimens. The fatigue strength on 50% failure probability at 107 cycles under the hydrogen-charging was slightly lower than that under the hydrogen-free condition. On the surface of the non-charged specimen that was run-out at 107 cycles, a number of cracks up to ≈100 µm in length was observed. By contrast, in the hydrogen-charged specimen, no crack was observed after the fatigue testing. These results suggested that the hydrogen-charging had a negative influence on the fatigue limit as well as fatigue life of the alloy used in this study.
 Fig. 7 S-N curves. Fullsize ImageView full abstractDownload PDF (1684K) Full view HTML
Fig. 7 S-N curves. Fullsize ImageView full abstractDownload PDF (1684K) Full view HTML -
Kazuya Kubo, Hideaki Itoh, Etsuo Akiba, Akari HayashiArticle type: Regular Article
2020 Volume 84 Issue 3 Pages 99-108
Published: March 01, 2020
Released on J-STAGE: February 25, 2020
JOURNAL FREE ACCESS FULL-TEXT HTMLVarious Ti-Mn-V based ternary alloys, known as AB2 type hydrogen storage alloys, were prepared and evaluated in order to clarify their crystal structures and hydrogen absorption/desorption properties. As a result, Mn-rich alloys with C14 laves structure were found to show a small hysteresis loop on absorbing/desorbing hydrogen. Furthermore, the effect of doping 4th and 5th element atoms on Ti-Mn-V based ternary alloys were evaluated. Then, Mn-rich Zr doped Ti-V-Mn alloys, like Ti30−xZrxV15Mn50, revealed most effective hydrogen storage capacity. Finally, we have found that a cooling process of melted alloys is important for synthesizing more homogeneous alloys, and successfully developed Ti25Zr10V15Mn50 with a rapid solidification process as one of the most effective hydrogen storage materials.
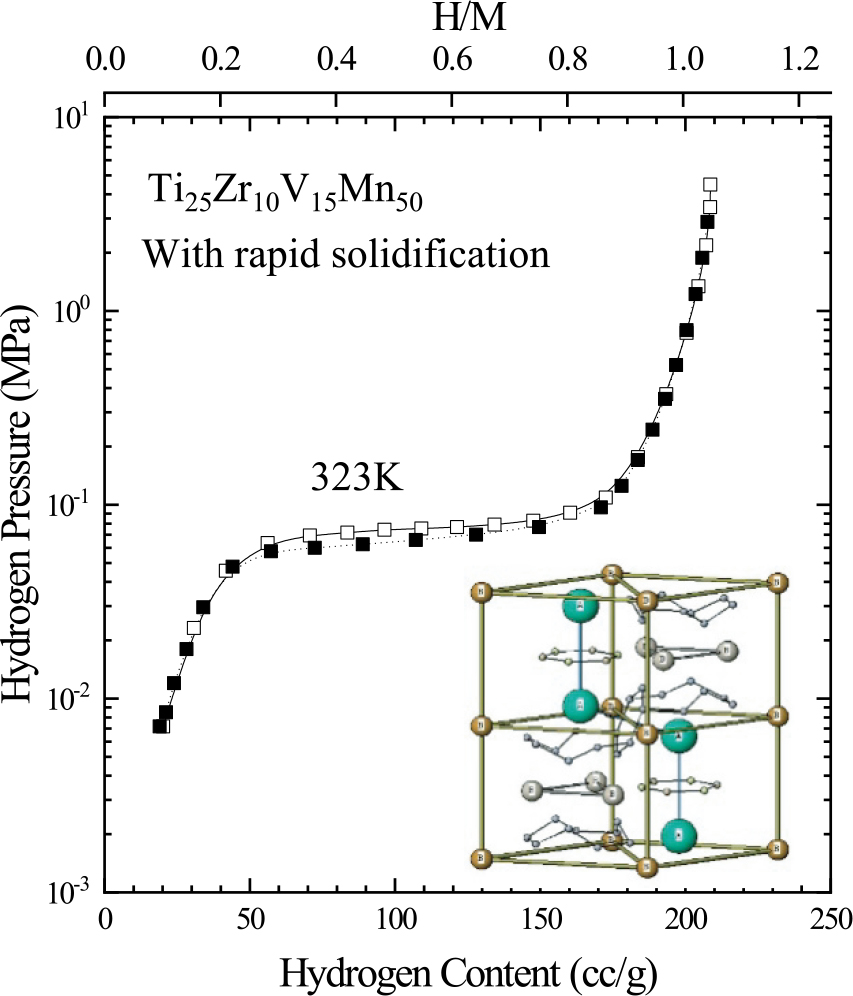 We successfully developed Ti25Zr10V15Mn50 with a rapid solidification process as one of the most effective hydrogen storage materials. Fullsize ImageView full abstractDownload PDF (8731K) Full view HTML
We successfully developed Ti25Zr10V15Mn50 with a rapid solidification process as one of the most effective hydrogen storage materials. Fullsize ImageView full abstractDownload PDF (8731K) Full view HTML -
Yoshiaki Hashimoto, Makoto Hino, Takehiko Yanagiya, Takeshi Yamaguchi, ...Article type: Regular Article
2020 Volume 84 Issue 3 Pages 109-114
Published: March 01, 2020
Released on J-STAGE: February 25, 2020
Advance online publication: February 07, 2020JOURNAL FREE ACCESS FULL-TEXT HTMLIn this study, the effects of carbon nanoparticles fixed to the surfaces of AM60B and AZ91D magnesium alloy chips on the mechanical properties were examined. The manufacture of magnesium-carbon alloy is not easy because carbon does not possess the property of wettability for magnesium. However, magnesium alloy chips fixed to carbon nanoparticles enable magnesium-carbon alloys to be produced by the thixomolding process. Mechanical properties such as the tensile and fatigue strengths were improved by only 0.1 mass% of the carbon addition because of the decrease in the void and the refinement of crystal grains. In addition, the AZ91D magnesium alloy was proven to be more effective than the AM60B magnesium alloy for the decrease in the void and the refinement of crystal grains by the carbon addition. The aluminum content of the AZ91D magnesium alloy is higher than that of the AM60B alloy. It seems that the void formation is based on the hydrogen by the reaction between aluminum in the magnesium alloy and water. Therefore, the effect of carbon addition on the mechanical properties was dependent on the aluminum content in the magnesium alloys.
 Fig. 5 Grain boundary maps obtained by EBSD analysis for AM60B and AZ91D alloy at R part. Fullsize ImageView full abstractDownload PDF (3574K) Full view HTML
Fig. 5 Grain boundary maps obtained by EBSD analysis for AM60B and AZ91D alloy at R part. Fullsize ImageView full abstractDownload PDF (3574K) Full view HTML
- |<
- <
- 1
- >
- >|